Abstract
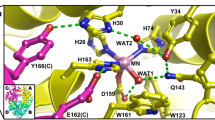
Manganese superoxide dismutase from the obligate thermophile Thermus thermophilus HB8 exhibits a thermal transition in azide complexes that resembles the behavior found for a mesophilic MnSD (from Escherichia coli) but shifted 85 degrees to higher temperature. The active-site structures of the two enzymes are virtually identical, yet the dynamical behavior is evidently distinct, apparently adapted to the physiological growth temperature for each organism. These results provide evidence for subtle tuning of structure for proteins that function in extreme physical environments.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 2 May 1997 / Accepted: 17 July 1997
Rights and permissions
About this article
Cite this article
Whittaker, M., Whittaker, J. A "thermophilic shift" in ligand interactions for Thermus thermophilus manganese superoxide dismutase. JBIC 2, 667–671 (1997). https://doi.org/10.1007/s007750050182
Issue Date:
DOI: https://doi.org/10.1007/s007750050182