Abstract
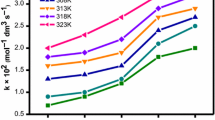
Kinetics of the steady-state oxidation of n–alkylferrocenes (alkyl = H, Me, Et, Bu and C5H11) by H2O2 to form the corresponding ferricenium cations catalyzed by horseradish peroxidase has been studied in micellar systems of Triton X-100, CTAB, and SDS, mostly at pH 6.0 and 25 °C. The rate of oxidation of ferrocenes with longer alkyl radicals is too slow to be measured. The reaction obeying the [RFc]:[H2O2] = 2 : 1 stoichiometry is strictly first-order in both HRP and RFc in a wide concentration range. The corresponding observed second-order rate constants k, which refer to the interaction of the peroxidase compound II (HRP-II) with RFc, decrease with the elongation of the alkyl substituent R, and this in turn is accompanied by an increase in the formal redox potentials E°′ in the same medium. Increasing the surfactant concentration lowers the rate constants k, the effect being due to the nonproductive binding of RFc to micelles rather than to enzyme inactivation. The micellar effects are accounted for in terms of the Berezin pseudo-phase model of micellar catalysis applied to the interaction of enzyme with organometallic substrates. The oxidation was found to occur primarily in the aqueous pseudo-phase and the calculated intrinsic second-order rate constants k w are (1.9 ± 0.5)×105, (2.7 ± 0.1)×104, and (5.9 ± 0.6)×103 M–1 s–1 for HFc, EtFc, and n–BuFc, respectively. The data obtained were used for estimating the self-exchange rate constants for the HRP-II/HRP couple in terms of the Marcus formalism.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 15 July 1996 / Accepted: 15 November 1996
Rights and permissions
About this article
Cite this article
Ryabov, A., Goral, V. Steady-state kinetics, micellar effects, and the mechanism of peroxidase-catalyzed oxidation of n -alkylferrocenes by hydrogen peroxide. JBIC 2, 182–190 (1997). https://doi.org/10.1007/s007750050123
Issue Date:
DOI: https://doi.org/10.1007/s007750050123