Abstract
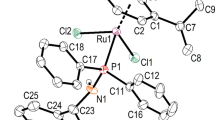
Five cationic ruthenium–arene complexes with the generic formula [Ru(SAc)(S2C·NHC)(p-cymene)](PF6) (5a–e) were prepared in almost quantitative yields using a straightforward one-pot, two-step experimental procedure starting from [RuCl2(p-cymene)]2, an imidazol(in)ium-2-dithiocarboxylate (NHC·CS2) zwitterion, KSAc, and KPF6. These half-sandwich compounds were fully characterized by various analytical techniques and the molecular structures of two of them were solved by X-ray diffraction analysis, which revealed the existence of an intramolecular chalcogen bond between the oxygen atom of the thioacetate ligand and a proximal sulfur atom of the dithiocarboxylate unit. DFT calculations showed that the C=S…O charge transfer amounted to 2.4 kcal mol−1. The dissolution of [Ru(SAc)(S2C·IMes)(p-cymene)](PF6) (5a) in moist DMSO-d6 at room temperature did not cause the dissociation of its sulfur ligands. Instead, p-cymene was slowly released to afford the 12-electron [Ru(SAc)(S2C·IMes)]+ cation that could be detected by mass spectrometry. Monitoring the solvolysis process by 1H NMR spectroscopy showed that more than 22 days were needed to fully decompose the starting ruthenium–arene complex. Compounds 5a–e exhibited a high antiproliferative activity against human glioma Hs683 and human lung carcinoma A549 cancer cells. In particular, the IMes derivative (5a) was the most potent compound of the series, achieving toxicities similar to those displayed by marketed platinum drugs.
Graphical abstract











Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the published article and its Supplementary Information files.
References
Collier WA, Krauss F (1931) Zur experimentellen Therapie der Tumoren. Z Krebsforsch 34:526–530. https://doi.org/10.1007/bf01625394
Dwyer FP, Gyarfas EC, Rogers WP, Koch JH (1952) Biological activity of complex ions. Nature 170:190–191. https://doi.org/10.1038/170190a0
Süss-Fink G (2010) Arene ruthenium complexes as anticancer agents. Dalton Trans 39:1673–1688. https://doi.org/10.1039/b916860p
Ang WH, Casini A, Sava G, Dyson PJ (2011) Organometallic ruthenium-based antitumor compounds with novel modes of action. J Organomet Chem 696:989–998. https://doi.org/10.1016/j.jorganchem.2010.11.009
Noffke AL, Habtemariam A, Pizarro AM, Sadler PJ (2012) Designing organometallic compounds for catalysis and therapy. Chem Commun 48:5219–5246. https://doi.org/10.1039/c2cc30678f
Nazarov AA, Hartinger CG, Dyson PJ (2014) Opening the lid on piano-stool complexes: an account of ruthenium(II)–arene complexes with medicinal applications. J Organomet Chem 751:251–260. https://doi.org/10.1016/j.jorganchem.2013.09.016
Singh SK, Pandey DS (2014) Multifaceted half-sandwich arene-ruthenium complexes: interactions with biomolecules, photoactivation, and multinuclearity approach. RSC Adv 4:1819–1840. https://doi.org/10.1039/c3ra44131h
Blunden BM, Stenzel MH (2015) Incorporating ruthenium into advanced drug delivery carriers—an innovative generation of chemotherapeutics. J Chem Technol Biotechnol 90:1177–1195. https://doi.org/10.1002/jctb.4507
Mari C, Pierroz V, Ferrari S, Gasser G (2015) Combination of Ru(II) complexes and light: new frontiers in cancer therapy. Chem Sci 6:2660–2686. https://doi.org/10.1039/c4sc03759f
Murray BS, Babak MV, Hartinger CG, Dyson PJ (2016) The development of RAPTA compounds for the treatment of tumors. Coord Chem Rev 306:86–114. https://doi.org/10.1016/j.ccr.2015.06.014
Thota S, Rodrigues DA, Crans DC, Barreiro EJ (2018) Ru(II) compounds: next-generation anticancer metallotherapeutics? J Med Chem 61:5805–5821. https://doi.org/10.1021/acs.jmedchem.7b01689
Meier-Menches SM, Gerner C, Berger W, Hartinger CG, Keppler BK (2018) Structure–activity relationships for ruthenium and osmium anticancer agents—towards clinical development. Chem Soc Rev 47:909–928. https://doi.org/10.1039/c7cs00332c
Marchetti F, Pettinari R, Di Nicola C, Pettinari C, Palmucci J, Scopelliti R, Riedel T, Therrien B, Galindo A, Dyson PJ (2018) Synthesis, characterization and cytotoxicity of arene–ruthenium(II) complexes with acylpyrazolones functionalized with aromatic groups in the acyl moiety. Dalton Trans 47:868–878. https://doi.org/10.1039/c7dt04249c
Swaminathan S, Haribabu J, Kalagatur NK, Konakanchi R, Balakrishnan N, Bhuvanesh N, Karvembu R (2019) Synthesis and anticancer activity of [RuCl2(η6-arene)(aroylthiourea)] complexes—high activity against the human neuroblastoma (IMR-32) cancer cell line. ACS Omega 4:6245–6256. https://doi.org/10.1021/acsomega.9b00349
Khan TA, Bhar K, Thirumoorthi R, Roy TK, Sharma AK (2020) Design, synthesis, characterization and evaluation of the anticancer activity of water-soluble half-sandwich ruthenium(II) arene halido complexes. New J Chem 44:239–257. https://doi.org/10.1039/c9nj03663f
Chen C, Xu C, Li T, Lu S, Luo F, Wang H (2020) Novel NHC-coordinated ruthenium(II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur J Med Chem 203:112605. https://doi.org/10.1016/j.ejmech.2020.112605
Harringer S, Wernitznig D, Gajic N, Diridl A, Wenisch D, Hejl M, Jakupec MA, Theiner S, Koellensperger G, Kandioller W, Keppler BK (2020) Introducing N-, P-, and S-donor leaving groups: an investigation of the chemical and biological properties of ruthenium, rhodium and iridium thiopyridone piano stool complexes. Dalton Trans 49:15693–15711. https://doi.org/10.1039/d0dt03165h
Marloye M, Inam H, Moore CJ, Debaille V, Pritchard JR, Gelbcke M, Meyer F, Dufrasne F, Berger G (2021) Synthesis, structure and anticancer properties of new biotin- and morpholine-functionalized ruthenium and osmium half-sandwich complexes. J Biol Inorg Chem 26:535–549. https://doi.org/10.1007/s00775-021-01873-9
Rafols L, Josa D, Aguilà D, Barrios L, Roubeau O, Cirera J, Soto-Cerrato V, Pérez-Tomás R, Martínez M, Grabulosa A, Gamez P (2021) Piano-stool ruthenium(II) complexes with delayed cytotoxic activity: origin of the lag time. Inorg Chem 60:7974–7990. https://doi.org/10.1021/acs.inorgchem.1c00507
Delaude L, Sauvage X, Demonceau A, Wouters J (2009) Synthesis and catalytic evaluation of ruthenium-arene complexes generated using imidazol(in)ium-2-carboxylates and -dithiocarboxylates. Organometallics 28:4056–4064. https://doi.org/10.1021/om9002363
Willem Q, Nicks F, Sauvage X, Delaude L, Demonceau A (2009) Ruthenium–arene complexes bearing imidazol(in)ium-2-dithiocarboxylate ligands: evaluation of their catalytic activity in the synthesis of enol esters. J Organomet Chem 694:4049–4055. https://doi.org/10.1016/j.jorganchem.2009.08.028
Delaude L (2009) Betaine adducts of N-heterocyclic carbenes: synthesis, properties, and reactivity. Eur J Inorg Chem. https://doi.org/10.1002/ejic.200801227
Beltrán TF, Delaude L (2017) Recent advances in small clusters and polymetallic assemblies based on transition metals and dithiocarboxylate zwitterions derived from N-heterocyclic carbenes. J Cluster Sci 28:667–678. https://doi.org/10.1007/s10876-017-1174-4
Delaude L, Demonceau A, Wouters J (2009) Assessing the potentials of zwitterionic NHC·CS2 adducts for probing the stereoelectronic parameters of N-heterocyclic carbenes. Eur J Inorg Chem. https://doi.org/10.1002/ejic.200801110
Mazars F, Hrubaru M, Tumanov N, Wouters J, Delaude L (2021) Synthesis of azolium-2-dithiocarboxylate zwitterions under mild, aerobic conditions. Eur J Org Chem. https://doi.org/10.1002/ejoc.202100274
Zain Aldin M, Zaragoza G, Deschamps W, Tomani J-CD, Souopgui J, Delaude L (2021) Synthesis, characterization, and biological activity of water-soluble, dual anionic and cationic ruthenium-arene complexes bearing imidazol(in)ium-2-dithiocarboxylate ligands. Inorg Chem 60:16769–16781. https://doi.org/10.1021/acs.inorgchem.1c02648
Wang F, Chen H, Parsons S, Oswald IDH, Davidson JE, Sadler PJ (2003) Kinetics of aquation and anation of ruthenium(II) arene anticancer complexes, acidity and X-ray structures of aqua adducts. Chem Eur J 9:5810–5820. https://doi.org/10.1002/chem.200304724
Wang F, Habtemariam A, van der Geer EPL, Fernández R, Melchart M, Deeth RJ, Aird R, Guichard S, Fabbiani FPA, Lozano-Casal P, Oswald IDH, Jodrell DI, Parsons S, Sadler PJ (2005) Controlling ligand substitution reactions of organometallic complexes: tuning cancer cell cytotoxicity. Proc Natl Acad Sci USA 102:18269. https://doi.org/10.1073/pnas.0505798102
Ang WH, Daldini E, Scolaro C, Scopelliti R, Juillerat-Jeannerat L, Dyson PJ (2006) Development of organometallic ruthenium-arene anticancer drugs that resist hydrolysis. Inorg Chem 45:9006–9013. https://doi.org/10.1021/ic061008y
Scolaro C, Hartinger CG, Allardyce CS, Keppler BK, Dyson PJ (2008) Hydrolysis study of the bifunctional antitumour compound RAPTA-C, [Ru(η6-p-cymene)Cl2(pta)]. J Inorg Biochem 102:1743–1748. https://doi.org/10.1016/j.jinorgbio.2008.05.004
Futera Z, Klenko J, Šponer JE, Šponer J, Burda JV (2009) Interactions of the “piano-stool” [ruthenium(II)(η6-arene)(en)Cl]+ complexes with water and nucleobases; ab initio and DFT study. J Comput Chem 30:1758–1770. https://doi.org/10.1002/jcc.21179
Zhao J, Zhang X, Liu H, Xiong Z, Li M, Chen T (2019) Ruthenium arene complex induces cell cycle arrest and apoptosis through activation of P53-mediated signaling pathways. J Organomet Chem 898:120869. https://doi.org/10.1016/j.jorganchem.2019.07.020
Lovison D, Allegri L, Baldan F, Ballico M, Damante G, Jandl C, Baratta W (2020) Cationic carboxylate and thioacetate ruthenium(II) complexes: synthesis and cytotoxic activity against anaplastic thyroid cancer cells. Dalton Trans 49:8375–8388. https://doi.org/10.1039/d0dt01390k
Al-Buthabhak HS, Falasca V, Yu Y, Sobolev AN, Skelton BW, Moggach SA, Ferro V, Al-Salami H, Baker MV (2022) Au-NHC complexes with thiocarboxylate ligands: synthesis, structure, stability, thiol exchange and in vitro anticancer activity. Appl Organomet Chem. https://doi.org/10.1002/aoc.6645
Furrer J, Süss-Fink G (2016) Thiolato-bridged dinuclear arene ruthenium complexes and their potential as anticancer drugs. Coord Chem Rev 309:36–50. https://doi.org/10.1016/j.ccr.2015.10.007
Păunescu E, Boubaker G, Desiatkina O, Anghel N, Amdouni Y, Hemphill A, Furrer J (2021) The quest of the best—a SAR study of trithiolato-bridged dinuclear ruthenium(II)-arene compounds presenting antiparasitic properties. Eur J Med Chem 222:113610. https://doi.org/10.1016/j.ejmech.2021.113610
Desiatkina O, Anghel N, Boubaker G, Amdouni Y, Hemphill A, Furrer J, Păunescu E (2023) Trithiolato-bridged dinuclear ruthenium(II)-arene conjugates tethered with lipophilic units: synthesis and Toxoplasma gondii antiparasitic activity. J Organomet Chem 986:122624. https://doi.org/10.1016/j.jorganchem.2023.122624
Bruker, (2005) APEX 3. Bruker AXS Inc., Madison
Clark RC, Reid JS (1995) The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr Sect A Fundam Crystallogr 51:887–897. https://doi.org/10.1107/s0108767395007367
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R (2005) SIR2004: an improved tool for crystal structure determination and refinement. J Appl Crystallogr 38:381–388. https://doi.org/10.1107/s002188980403225x
Sheldrick G (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8. https://doi.org/10.1107/s2053229614024218
Sheldrick GM (1996) SADABS, programs for scaling and correction of area detection data. University of Göttingen, Göttingen
Neese F (2012) The ORCA program system. Wiley Interdiscipl Rev Comput Mol Sci 2:73–78. https://doi.org/10.1002/wcms.81
Neese F (2018) Software update: the ORCA program system, version 4.0. Wiley Interdiscipl Rev Comput Mol Sci 8:e1327. https://doi.org/10.1002/wcms.1327
Mardirossian N, Head-Gordon M (2016) ωB97M-V: a combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J Chem Phys 144:214110. https://doi.org/10.1063/1.4952647
Mardirossian N, Ruiz Pestana L, Womack JC, Skylaris C-K, Head-Gordon T, Head-Gordon M (2017) Use of the rVV10 nonlocal correlation functional in the B97M-V density functional: defining B97M-rV and related functionals. J Phys Chem Lett 8:35–40. https://doi.org/10.1021/acs.jpclett.6b02527
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/b508541a
Weigend F (2006) Accurate Coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys 8:1057–1065. https://doi.org/10.1039/b515623h
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Karafiloglou P, Landis CR, Weinhold F (2018) NBO 70. Theoretical Chemistry Institute, University of Wisconsin, Madison
Syed Mohamed K, Padma DK (1985) Spectral studies on pyridinium hexafluorophosphate. Spectrochim Acta Part A 41:725–728. https://doi.org/10.1016/0584-8539(85)80181-1
Mayfield HG, Bull WE (1971) Co-ordinating tendencies of the hexafluorophosphate ion. J Chem Soc A. https://doi.org/10.1039/j19710002279
Only data for complexes 3a–e are displayed in Table 2, see Ref. 26 for complexes 4a–e
Bauzá A, Quiñonero D, Deyà PM, Frontera A (2013) Halogen bonding versus chalcogen and pnicogen bonding: a combined Cambridge structural database and theoretical study. CrystEngComm 15:3137–3144. https://doi.org/10.1039/c2ce26741a
Pascoe DJ, Ling KB, Cockroft SL (2017) The origin of chalcogen-bonding interactions. J Am Chem Soc 139:15160–15167. https://doi.org/10.1021/jacs.7b08511
Scilabra P, Terraneo G, Resnati G (2019) The chalcogen bond in crystalline solids: a world parallel to halogen bond. Acc Chem Res 52:1313–1324. https://doi.org/10.1021/acs.accounts.9b00037
Vogel L, Wonner P, Huber SM (2019) Chalcogen bonding: an overview. Angew Chem Int Ed 58:1880–1891. https://doi.org/10.1002/anie.201809432
Beno BR, Yeung K-S, Bartberger MD, Pennington LD, Meanwell NA (2015) A survey of the role of noncovalent sulfur interactions in drug design. J Med Chem 58:4383–4438. https://doi.org/10.1021/jm501853m
Mahmudov KT, Aliyeva VA, Guedes da Silva MFC, Pombeiro AJL (2021) The chalcogen bond in solution: synthesis, catalysis and molecular recognition. In: Huber SM (ed) Halogen bonding in solution. Wiley-VCH, Weinheim, pp 363–382. https://doi.org/10.1002/9783527825738.ch11
Vydrov OA, Van Voorhis T (2010) Nonlocal van der Waals density functional: the simpler the better. J Chem Phys 133:244103. https://doi.org/10.1063/1.3521275
Iron MA, Janes T (2019) Evaluating transition metal barrier heights with the latest density functional theory exchange-correlation functionals: the MOBH35 benchmark database. J Phys Chem A 123:3761–3781. https://doi.org/10.1021/acs.jpca.9b01546
Iron MA, Janes T (2019) Correction to “Evaluating transition metal barrier heights with the latest density functional theory exchange-correlation functionals: the MOBH35 benchmark database.” J Phys Chem A 123:6379–6380. https://doi.org/10.1021/acs.jpca.9b06135
Berger G, Leclercqz H, Derenne A, Gelbcke M, Goormaghtigh E, Nève J, Mathieu V, Dufrasne F (2014) Synthesis and in vitro characterization of platinum(II) anticancer coordinates using FTIR spectroscopy and NCI COMPARE: a fast method for new compound discovery. Bioorg Med Chem 22:3527–3536. https://doi.org/10.1016/j.bmc.2014.04.017
Acknowledgements
The authors would like to thank Dr. Johann Far for the ESI-MS analyses, Mr Stéphane Luts and Prof. Gauthier Eppe for the FT-IR analyses, and RIAIDT-USC for the use of its analytical facilities.
Funding
Financial support from the Fonds de la Recherche Scientifique—FNRS under CDR Grants J.0155.18 and J.0013.23 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zain Aldin, M., Zaragoza, G., Choquenet, E. et al. Synthesis, characterization, and biological activity of cationic ruthenium–arene complexes with sulfur ligands. J Biol Inorg Chem (2024). https://doi.org/10.1007/s00775-024-02052-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00775-024-02052-2