Abstract
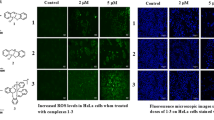
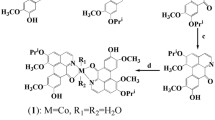
The adverse side effects and acquired resistance associated with the clinical application of traditional platinum-based anticancer drugs have forced investigation of alternative transition metal-based compounds and their cytostatic properties. Over the last years, the anticancer potential of cobalt complexes has been extensively studied, and in-depth analyses of their mode of action have been conducted. In this work, we present antiproliferative activity against human cancer cells of the dinuclear Co(III) complexes bearing the quinizarin ligand and tris(2-aminoethyl)amine (tren, compound 1) or tris(2-pyridylmethyl)amine (tpa, compound 2) co-ligands. To contribute the understanding mechanisms of biological action of these compounds, their association with DNA in the cells, DNA binding in cell-free media, and DNA cleavage capability were investigated in detail. The results demonstrate that both complexes interact with DNA in tumor cells. However, their mechanism of antiproliferative action is different, and this difference is mirrored by distinct antiproliferative activity. The antiproliferative effect of 1 is connected with its ability to intercalate into DNA and subsequently to inhibit activities of DNA processing enzymes. In contrast, the total antiproliferative efficiency of 2, thanks to its redox properties, appears to be connected with its ability to form radicals and, consequently, with the ability of 2 to cleave DNA. Hence, the findings presented in this study may significantly contribute to understanding the antitumor potential of cobalt complexes.
Graphic abstract
Dinuclear Co(III) complexes containing the bioactive quinizarin ligand exhibit antiproliferative activity based on distinct mechanism










Similar content being viewed by others
References
Johnstone TC, Suntharalingam K, Lippard SJ (2016) Chem Rev 116:3436–3486
Hall MD, Failes TW, Yamamoto N, Hambley TW (2007) Dalton Trans 36:3983–3990
Heffern MC, Yamamoto N, Holbrook RJ, Eckermann AL, Meade TJ (2013) Curr Opin Chem Biol 17:189–196
Munteanu CR, Suntharalingam K (2015) Dalton Trans 44:13796–13808
Ahn GO, Botting KJ, Patterson AV, Ware DC, Tercel M, Wilson WR (2006) Biochem Pharmacol 71:1683–1694
Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG, Hambley TW (2007) Chem Eur J 13:2974–2982
Bonnitcha PD, Kim BJ, Hocking RK, Clegg JK, Turner P, Neville SM, Hambley TW (2012) Dalton Trans 41:11293–11304
Ahmad M, Afzal M, Tabassum S, Kalińska B, Mrozinski J, Bharadwaj PK (2014) Eur J Med Chem 74:683–693
Massoud SS, Perkins RS, Louka FR, Xu W, Le Roux A, Dutercq Q, Fischer RC, Mautner FA, Handa M, Hiraoka Y, Kreft GL, Bortolotto T, Terenzi H (2014) Dalton Trans 43:10086–10103
Hengstler JG, Bolm-Audorff U, Faldum A, Janssen K, Reifenrath M, Götte W, Jung D, Mayer-Popken O, Fuchs J, Gebhard S, Bienfait HG, Schlink K, Dietrich C, Faust D, Epe B, Oesch F (2003) Carcinogenesis 24:63–73
Stenger C, Naves T, Verdier M, Ratinaud M (2011) Int J Oncol 39:601–609
He Y, Gan X, Zhang L, Liu B, Zhu Z, Li T, Zhu J, Chen J, Yu H (2018) Am J Physiol Cell Physiol 315:C389–C397
Cressey PB, Eskandari A, Bruno PM, Lu CX, Hemann MT, Suntharalingam K (2016) ChemBioChem 17:1713–1718
Cressey PB, Eskandari A, Suntharalingam K (2017) Inorganics 5:12
Eskandari A, Kundu A, Lu C, Ghosh S, Suntharalingam K (2018) Dalton Trans 47:5755–5763
Renfrew AK (2014) Metallomics 6:1324–1335
de Souza ICA, Faro LV, Pinheiro CB, Gonzaga DTG, da Silva FdC, Ferreira VF, Miranda FdS, Scarpellini M, Lanznaster M (2016) Dalton Trans 45:13671–13674
Kozsup M, Dömötör O, Nagy S, Farkas E, Enyedy EA, Buglyó P (2019) J Inorg Biochem. https://doi.org/10.1016/jinogbio.2019.110963
Batchelor-McAuley C, Dimov IB, Aldous L, Compton RG (2011) Proc Natl Acad Sci USA 108:19891–19895
Verebová V, Adamcik J, Danko P, Podhradský D, Miškovský P, Staničová J (2014) Biochem Biophys Res Commun 444:50–55
Gholivand MB, Kashanian S, Peyman H (2012) Spectrochim Acta Part A 87:232–240
Rossi S, Tabolacci C, Lentini A, Provenzano B, Carlomosti F, Frezzotti S, Beninati S (2010) Anticancer Res 30:445–449
Zengin G, Degirmenci N, Alpsoy L, Aktumsek A (2016) Hum Exp Toxicol 35:544–553
Lee H-S (2003) J Microbiol Biotechnol 13:529–536
Gholivand MB, Kashanian S, Peyman H, Roshanfekr H (2011) Eur J Med Chem 46:2630–2638
Ghosh P, Devi GP, Priya R, Amrita A, Sivaramakrishna A, Babu S, Siva R (2013) Appl Biochem Biotechnol 170:1127–1137
Lozano HJ, Busto N, Espino G, Carbayo A, Leal JM, Platts JA, Garcia B (2017) Dalton Trans 46:3611–3622
Kostrhunova H, Zajac J, Novohradsky V, Kasparkova J, Malina J, Aldrich-Wright JR, Petruzzella E, Sirota R, Gibson D, Brabec V (2019) J Med Chem 62:5176–5190
Malina J, Čechová K, Farrell NP, Brabec V (2019) Inorg Chem 58:6804–6810
Pracharova J, Radosova Muchova T, Dvorak Tomastikova E, Intini FP, Pacifico C, Natile G, Kasparkova J, Brabec V (2016) Dalton Trans 45:13179–13186
Novohradsky V, Zajac J, Vrana O, Kasparkova J, Brabec V (2018) Oncotarget 9:28456–28473
Subramanian A, Kumar YM, Arunachalam S, Gowdhami B, Sundaram K, Solomon V, Venuvanalingam P, Akbarsha MA, Sundararaman M (2019) Sci Rep 9:2721
Palchaudhuri R, Hergenrother PJ (2007) Curr Opin Biotechnol 18:497–503
Norden B, Kubista M, Kurucsev T (1992) Q Rev Biophys 25:51–170
Rodger A, Marington R, Geeves MA, Hicks M, de Alwis L, Halsall DJ, Dafforn TR (2006) Phys Chem Chem Phys 8:3161–3171
Satyanarayana S, Dabrowiak JC, Chaires JB (1992) Biochemistry 31:9319–9324
Ihara T, Ikegami T, Fujii T, Kitamura Y, Sueda S, Takagi M, Jyo A (2006) J Inorg Biochem 100:1744–1754
Drwal MN, Agama K, Wakelin LPG, Pommier Y, Griffith R (2011) PLoS One 6:e25150
Chamaon K, Schoenfeld P, Awiszus F, Bertrand J, Lohmann C (2018) J Biomed Mater Res Part B 107:1246–1253
Lan AP, Chen J, Chai ZF, Hu Y (2016) Biometals 29:665–678
Acknowledgements
This work was supported by the Czech Science Foundation (Grant no. 18-09502S). The work of H. C. and J. P. was supported by the (Project IGA_PrF_2019_030). The research of MK, SN, and PB was supported by the EU and co-financed by the European Regional Development Fund under the project GINOP-2.3.2-15-2016-00008, the Hungarian Scientific Research Fund (OTKA K112317), and the ÚNKP-19-3-I-DE-45, ÚNKP-19-3-I-DE-56 New National Excellence Program of the Ministry for Innovation and Technology (Hungary).
Author information
Authors and Affiliations
Contributions
JK designed research; HC, HK, JP, and JK performed experiments and analyzed data; MK, SN, and PB supplied cobalt complexes and measured NMR spectra; VB and JK wrote the manuscript; all authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crlikova, H., Kostrhunova, H., Pracharova, J. et al. Antiproliferative, DNA binding, and cleavage properties of dinuclear Co(III) complexes containing the bioactive quinizarin ligand. J Biol Inorg Chem 25, 339–350 (2020). https://doi.org/10.1007/s00775-020-01765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01765-4