Abstract
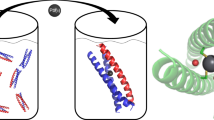
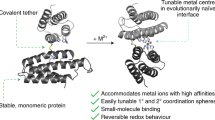
We use a de Novo protein design strategy to demonstrate that the second coordination sphere of a metal site plays a key role in controlling coordination geometries of Cd(II)-tris-thiolate complexes. Specifically, we show that alteration of chirality within the core hydrophobic packing region of a three-stranded coiled coil (3SCC) can control the coordination number of Cd(II) by limiting steric encumbrance to the metal center. Within a specific class of 3SCCs [Ac-G-(LKALEEK) n -G-NH2], where n = 4 is TRI and n = 5 is GRAND, one l-Leu may be substituted by l-Cys to generate a planar tris-thiolate array capable of metal binding. In the native peptide containing only the l-configuration of leucine, the three-Cys ligand site leads to a mixture of 3- and 4-coordinate Cd(II). When the l-Leu above (toward the N-terminus) the tris-Cys site is substituted with d-Leu, solely a 3-coordinate structure [Cd(II)S3] was obtained. When d-Leu is located below (toward the C-terminus), a mixture of two coordination geometries, presumably Cd(II)S3O and Cd(II)S3O2, is observed, while substitution with d-Leu both above and below the tris-Cys plane yields a higher percentage of 4-coordinate Cd(II)S3O species. Thus, the use of d-amino acids around a metal’s coordination sphere provides a powerful tool for controlling the properties of future designed metalloproteins.






Similar content being viewed by others
References
Hu C, Wang J (2016) Method for enzyme design with genetically encoded unnatural amino acids. In: Pecoraro VL (ed) Methods in enzymology, vol 580 “peptide, protein and enzyme design”, vol 580. Elsevier, United States, pp 109–134
Guo JT, Wang JY, Lee JS, Schultz PG (2008) Angew Chem 47:6399–6401
Liu CC, Schultz PG (2010) Ann Rev Biochem 79:413–444
Peacock AFA, Hemmingsen L, Pecoraro VL (2008) Proc Natl Acad Sci USA 105(43):16566–16571
Peacock AFA, Stuckey JA, Pecoraro VL (2009) Angew Chem Int Ed Engl 48(40):7371–7374
Lee K-H, Cabello C, Hemmingsen L, Marsh ENG, Pecoraro VL (2006) Angew Chem Int Ed Engl 45(18):2864–2868
Petros AK, Shaner SE, Costello AL, Tierney DL, Gibney BR (2004) Inorg Chem 43(16):4793–4795
Lu Y (2005) Curr Opin Chem Biol 9(2):118–126
Kumar A, Ramakrishnan V (2010) ACS Synth Biol 4(4):221–247
Baures PW, Ojala WH, Gleason WB, Johnson RL (1997) J Pept Res 50(1):1–13
Wang A, Nairn NW, Marelli M, Grabstein K (2012) In: Kaumaya PP (ed) Protein engineering. In Tech, Croatia, pp 253–290
Privett HK, Reedy CJ, Kennedy ML, Gibney BR (2002) J Am Chem Soc 124(24):6828–6829
Zastrow ML, Peacock AFA, Stuckey JA, Pecoraro VL (2012) Nat Chem 4:118–123
Balaram P (1999) J Pept Res 54(3):195–199
Mahalakshmi R, Balaram P (2006) A new frontier in amino acid and protein research. Nova Science Publishers Inc, New York, pp 416–430
Dhanasekaran M, Fabiola F, Pattabhi V, Durani S (1999) J Am Chem Soc 121:5575–5576
Rana S, Kundu B, Durani S (2004) Chem Commun (Camb) 21:2462–2463
Rana S, Kundu B, Durani S (2005) Chem Commun (Camb) 2:207–209
Rana S, Kundu B, Durani S (2007) Bioorg Med Chem 15(11):3874–3882
Rana S, Kundu B, Durani S (2007) Biopolymer 87(4):231–243
Petal K, Srivastava KR, Durani S (2010) Bioinorg Med Chem 18:8270–8276
Ramachandran GN, Chandrasekaran R (1972) Indian J Biochem Biophys 9:1–11
Ghadiri MR, Granja JR, Buehler LK (1994) Nature 369(6478):301–304
Fernandez-Lopez S, Kim HS, Choi EC, Delgado M, Granja JR, Khasanov A, Kraehenbuehl K, Long G, Weinberger DA, Wilcoxen KM, Ghadiri MR (2001) Nature 412:452–456
Aravinda S, Shamala N, Pramanik A, Das C, Balaram P (2000) Biochem Biophys Res Commun 273(3):933–936
Aravinda S, Shamala N, Bandyopadhyay A, Balaram P (2003) J Am Chem Soc 125(49):15065–15075
Ruckthong L, Peacock AFA, Pascoe CE, Hemmingsen L, Stuckey JA, Pecoraro VL (2017) Chemistry 23(34):8232–8243
Dieckmann GR, McRorie DK, Lear JD, Sharp KA, DeGrado WF, Pecoraro VL (1998) J Mol Biol 280(5):897–912
Dieckmann GR, Mcrorie DK, Tierney DL, Utschig LM, Singer CP, O’Halloran TV, Penner-Hahn JE, Degrado WF, Pecoraro VL (1997) J Am Chem Soc 119(4):6195–6196
Ghosh D, Pecoraro VL (2004) Inorg Chem 43(25):7902–7915
Farrer BT, McClure CP, Penner-Hahn JE, Pecoraro VL (2000) Inorg Chem 39(24):5422–5423
Touw DS, Nordman CE, Stuckey JA, Pecoraro VL (2007) Proc Natl Acad Sci USA 104(29):11969–11974
Chakraborty S, Touw DS, Peacock AFA, Stuckey J, Pecoraro VL (2010) J Am Chem Soc 132(38):13240–13250
Matzapetakis M, Pecoraro VL (2005) J Am Chem Soc 127(51):18229–18233
Matzapetakis M, Farrer BT, Weng T-C, Hemmingsen L, Penner-Hahn JE, Pecoraro VL (2002) J Am Chem Soc 124(27):8042–8054
Matzapetakis M, Ghosh D, Weng T-C, Penner-Hahn JE, Pecoraro VL (2006) J Biol Inorg Chem 11(7):876–890
Lee K-H, Matzapetakis M, Mitra S, Marsh ENG, Pecoraro VL (2004) J Am Chem Soc 126(30):9178–9179
Zampella G, Neupane KP, De Gioia L, Pecoraro VL (2012) Eur J Inorg Chem 18(7):2040–2050
Neupane KP, Pecoraro VL (2010) Angew Chem Int Ed Engl 49(44):8177–8180
Iranzo O, Thulstrup PW, Ryu S-B, Hemmingsen L, Pecoraro VL (2007) Chemistry 13(33):9178–9190
Iranzo O, Jakusch T, Lee K-H, Hemmingsen L, Pecoraro VL (2009) Chemistry 15(15):3761–3772
Ruckthong L, Zastrow ML, Stuckey JA, Pecoraro VL (2016) J Am Chem Soc 138(36):11979–11988
Iranzo O, Cabello C, Pecoraro VL (2007) Angew Chem Int Ed Engl 46(35):6688–6691
Dieckmann G, Heilman S, DeGrado W, Pecoraro VL (1995) De novo design of metallopeptides. In: Kessissoglou DP, Coucouvanis D, Kanatzidas M (eds) An inorganic perspective of life. Elsevier, Amsterdam, p 275
Pecoraro VL, Peacock AFA, Iranzo O, Marek Ł (2009) Understanding the biological chemistry of mercury using a de novo protein design strategy. In: Long E et al. (eds) Bioinorganic Chemistry, ACS Symposium Series; American Chemical Society, Washington, DC, pp 183–197
Farrer BT, Pecoraro VL (2003) Proc Natl Acad Sci USA 100(7):3760–3765
Iranzo O, Chakraborty S, Hemmingsen L, Pecoraro VL (2011) J Am Chem Soc 133(2):239–251
Coleman JE (1993) Methods Enzymol 227:16–43
Summers F (1988) Coord Chem Rev 86:43–134
Oz G, Pountney DL, Armitage IM (1998) Biochem Cell Biol 76(2–3):223–234
Hemmingsen L, Olsen L, Antony J, Sauer SPA (2004) J Biol Inorg Chem 9(5):591–599
Hemmingsen L, Stachura M, Thulstrup PW, Christensen NJ, Johnston K (2010) Hyperfine Interact 197(1–3):255–267
Hemmingsen L, Sas KN, Danielsen E (2004) Chem Rev 104(9):4027–4062
Farrer BT, Harris NP, Balchus KE, Pecoraro VL (2001) Biochemistry 40(48):14696–14705
Ellman GL (1959) Arch Biochem Biophys 82:70–77
Cobas C, Cruces J, Sardina FJ (2000) In: 2.3 edn. Universidad de Santiago de Compostela, Santiago de Compostela
Hemmingsen L, Bauer R, Bjerrum M, Adolph H, Zeppezauer M, Cedergren-Zeppezauer E (1996) Eur J Biochem 241:546–551
McMaster WH, Del Grande NK, Mallett JH, Hubbell JH (1969) Lawrence Livermore Radiation Laboratory, Livermore
Duhme AK, Strasdeit H (1999) Z Anorg Allg Chem 625:6–8
Clark-Baldwin K, Tierney D, Govindaswamy N, Gruff ES, Kim C, Berg J, Koch SA, Penner-Hahn JE (1998) J Am Chem Soc 120:8401–8409
Ruckthong L (2016) Dissertation, University of Michigan, Ann Arbor
Chen YH, Yang JT, Chau KH (1974) Biochemistry 13:3350–3359
Dieckmann GR (1995) Dissertation, University of Michigan, Ann Arbor
Shannon RD (1976) Acta Crystallogr A 32:751–767
Ghosh D, Lee K-H, Demeler B, Pecoraro VL (2005) Biochemistry 44(31):10732–10740
Santos RA, Gruff ES, Koch SA, Harbison GS (1991) J Am Chem Soc 5:469–475
Acknowledgements
This manuscript is dedicated to Prof. Dr. Helmut Sigel on the occasion of his 80th birthday. Prof. Sigel’s contributions over 5 decades have been immense. His description of metal interactions with nucleotides has been groundbreaking and authoritative, while his inexhaustible energy to the community with his book series and service roles to the Society of Biological Inorganic Chemistry and the International Conference on Biological Inorganic Chemistry has helped place the discipline of bioinorganic chemistry as a major field of scientific endeavor. The authors acknowledge funding from the National Institutes of Health (L.R. and V.L.P., ES012236; A.D. and J.E.P.-H., GM 38047) for support of this research. Synchrotron measurements were made at the Stanford Synchrotron Radiation Laboratory, which is supported by the NIH Research Resource Program and the US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruckthong, L., Deb, A., Hemmingsen, L. et al. Incorporation of second coordination sphere d-amino acids alters Cd(II) geometries in designed thiolate-rich proteins. J Biol Inorg Chem 23, 123–135 (2018). https://doi.org/10.1007/s00775-017-1515-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1515-7