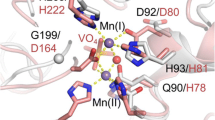
Abstract
The metallo-β-lactamase (MβL) superfamily is a functionally diverse group of metalloproteins sharing a distinctive αβ/αβ fold and a characteristic metal binding motif. A large number of open reading frames identified in genomic sequencing efforts have been annotated as members of this superfamily through sequence comparisons. However, structural and functional studies performed on purified proteins are normally needed to unequivocally include a newly discovered protein in the MβL superfamily. Here we report the spectroscopic characterization of recombinant YcbL, a gene product annotated as a member of the MβL superfamily whose function in vivo remains unknown. By taking advantage of the structural features characterizing the MβL superfamily metal binding motif, we performed spectroscopic studies on Zn(II)- and Co(II)-substituted YcbL to structurally interrogate the metal binding site. The dinuclear center in Co(II)-YcbL was shown to display characteristic electronic absorption features in the visible region, which were also observed in an engineered MβL aimed at mimicking this metal site. Thus, the spectroscopic features reported herein can be employed as a signature to readily identify and characterize the presence of these ubiquitous metal binding sites.





Similar content being viewed by others
References
Daiyasu H, Osaka K, Ishino Y, Toh H (2001) FEBS Lett 503:1–6
Gomes CM, Frazao C, Xavier AV, LeGall J, Teixeira M (2002) Protein Sci 11:707–712
Carfi A, Pares S, Duee E, Galleni M, Duez C, Frère JM, Dideberg O (1995) EMBO J 14:4914–4921
Campos-Bermudez VA, Leite NR, Krog R, Costa-Filho AJ, Soncini FC, Oliva G, Vila AJ (2007) Biochemistry 46:11069–11079
Andreini C, Banci L, Bertini I, Rosato A (2006) J Proteome Res 5:3173–3178
Alfredson DA, Korolik V (2007) FEMS Immunol Med Microbiol 49:159–164
Bebrone C (2007) Biochem Pharmacol 74:1686–1701
Periyannan GR, Costello AL, Tierney DL, Yang KW, Bennett B, Crowder MW (2006) Biochemistry 45:1313–1320
Llarrull LI, Tioni MF, Kowalski J, Bennett B, Vila AJ (2007) J Biol Chem 282:30586–30595
McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK (2001) Nature 413:852–856
Checa SK, Espariz M, Audero ME, Botta PE, Spinelli SV, Soncini FC (2007) Mol Microbiol 63:1307–1318
Sharrocks AD (1994) Gene 138:105–108
Gonzalez JM, Medrano Martin FJ, Costello AL, Tierney DL, Vila AJ (2007) J Mol Biol 373:1141–1156
Gill SC, von Hippel PH (1989) Anal Biochem 182:319–326
Paul-Soto R, Bauer R, Frere JM, Galleni M, Meyer-Klaucke W, Nolting H, Rossolini GM, de Seny D, Hernandez-Valladares M, Zeppezauer M, Adolph HW (1999) J Biol Chem 274:13242–13249
Hunt JB, Neece SH, Ginsburg A (1985) Anal Biochem 146:150–157
Thomas PW, Stone EM, Costello AL, Tierney DL, Fast W (2005) Biochemistry 44:7559–7569
Ankudinov AL, Ravel B, Rehr JJ, Conradson SD (1998) Phys Rev B 58:7565–7576
McClure CP, Rusche KM, Peariso K, Jackman JE, Fierke CA, Penner-Hahn JE (2003) J Inorg Biochem 94:78–85
Costello A, Periyannan G, Yang KW, Crowder MW, Tierney DL (2006) J Biol Inorg Chem 11:351–358
Siemann S, Brewer D, Clarke AJ, Dmitrienko GI, Lajoie G, Viswanatha T (2002) Biochim Biophys Acta 1571:190–200
Kuzmic P (1996) Anal Biochem 237:260–273
Inubushi T, Becker ED (1983) J Magn Reson 51:128–133
Bertini I, Turano P, Vila AJ (1993) Chem Rev 93:2833–2932
Marasinghe GP, Sander IM, Bennett B, Periyannan G, Yang KW, Makaroff CA, Crowder MW (2005) J Biol Chem 280:40668–40675
Garcia-Saez I, Mercuri PS, Papamicael C, Kahn R, Frere JM, Galleni M, Rossolini GM, Dideberg O (2003) J Mol Biol 325:651–660
Sali A, Blundell TL (1993) J Mol Biol 234:779–815
Laskowski RA, Mac Arthur MW, Moss DS, Thornton JM (1993) J Appl Crystallogr 26:283–291
Schilling O, Wenzel N, Naylor M, Vogel A, Crowder M, Makaroff C, Meyer-Klaucke W (2003) Biochemistry 42:11777–11786
Vogel A, Schilling O, Meyer-Klaucke W (2004) Biochemistry 43:10379–10386
Dong YJ, Bartlam M, Sun L, Zhou YF, Zhang ZP, Zhang CG, Rao Z, Zhang XE (2005) J Mol Biol 353:655–663
Cameron AD, Ridderstrom M, Olin B, Mannervik B (1999) Structure 7:1067–1078
Bertini I, Luchinat C, Viezzoli MS (1986) In: Bertini I, Luchinat C, Maret W, Zeppezauer M (eds) Zinc enzymes. Birkhauser, Boston
Bertini I, Luchinat C (1985) Adv Inorg Biochem 6:71–111
de Seny D, Heinz U, Wommer S, Kiefer M, Meyer-Klaucke W, Galleni M, Frere JM, Bauer R, Adolph HW (2001) J Biol Chem 276:45065–45078
Liu D, Thomas PW, Momb J, Hoang QQ, Petsko GA, Ringe D, Fast W (2007) Biochemistry 46:11789–11799
Kostelecky B, Pohl E, Vogel A, Schilling O, Meyer-Klaucke W (2006) J Bacteriol 188:1607–1614
Ishii R, Minagawa A, Takaku H, Takagi M, Nashimoto M, Yokoyama S (2005) J Biol Chem 280:14138–14144
Vila AJ, Fernandez CO (1996) J Am Chem Soc 118:7291–7298
Orellano EG, Girardini JE, Cricco JA, Ceccarelli EA, Vila AJ (1998) Biochemistry 37:10173–10180
McGuffin LJ, Jones DT (2003) Bioinformatics 19:874–881
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
Acknowledgments
This work was supported by grants from ANPCyT and HHMI to A.J.V. V.C.B is a recipient of a fellowship from CONICET. A.J.V. is a staff member from CONICET and an International Research Scholar of the Howard Hughes Medical Institute. The 600 MHz NMR spectrometer was purchased with funds from ANPCyT (PME2003-0026) and CONICET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campos-Bermudez, V.A., González, J.M., Tierney, D.L. et al. Spectroscopic signature of a ubiquitous metal binding site in the metallo-β-lactamase superfamily. J Biol Inorg Chem 15, 1209–1218 (2010). https://doi.org/10.1007/s00775-010-0678-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-010-0678-2