Abstract
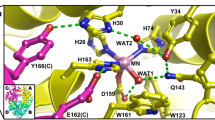
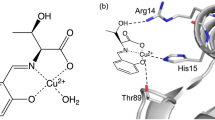
Nickel superoxide dismutase (NiSOD) is unique among the family of superoxide dismutase enzymes in that it coordinates Cys residues (Cys2 and Cys6) to the redox-active metal center and exhibits a hexameric quaternary structure. To assess the role of the Cys residues with respect to the activity of NiSOD, mutations of Cys2 and Cys6 to Ser (C2S-NiSOD, C6S-NiSOD, and C2S/C6S-NiSOD) were carried out. The resulting mutants do not catalyze the disproportionation of superoxide, but retain the hexameric structure found for wild-type NiSOD and bind Ni(II) ions in a 1:1 stoichiometry. X-ray absorption spectroscopic studies of the Cys mutants revealed that the nickel active-site structure for each mutant resembles that of C2S/C6S-NiSOD and demonstrate that mutation of either Cys2 or Cys6 inhibits coordination of the remaining Cys residue. Mutation of one or both Cys residue(s) in NiSOD induces the conversion of the low-spin Ni(II) site in the native enzyme to a high-spin Ni(II) center in the mutants. This result indicates that coordination of both Cys residues is required to generate the native low-spin configurations and maintain catalytic activity. Analysis of the quaternary structure of the Cys mutants by differential scanning calorimetry, mass spectrometry, and size-exclusion chromatography revealed that the Cys ligands, particularly Cys2, are also important for stabilizing the hexameric quaternary structure of the native enzyme.







Similar content being viewed by others
Abbreviations
- CuZnSOD:
-
Copper- and zinc-containing superoxide dismutase
- DFT:
-
Density functional theory
- DSC:
-
Differential scanning calorimetry
- EPR:
-
Electron paramagnetic resonance
- ESI-MS:
-
Electrospray ionization mass spectrometry
- EXAFS:
-
Extended X-ray absorption fine structure
- FeSOD:
-
Iron-containing superoxide dismutase
- LIC:
-
Ligation-independent cloning
- MnSOD:
-
Manganese-containing superoxide dismutase
- NHE:
-
Normal hydrogen electrode
- Ni–NTA:
-
Nickel nitrilotriacetic acid
- NiSOD:
-
Nickel-containing superoxide dismutase
- PCR:
-
Polymerase chain reaction
- SOD:
-
Superoxide dismutase
- Tris:
-
Tris(hydroxymethyl)aminomethane
- XANES:
-
X-ray absorption near-edge spectroscopy
- XAS:
-
X-ray absorption spectroscopy
References
Cabelli DE, Riley D, Rodriguez JA, Valentine JS, Zhu H (1998) In: Meunier B (eds) Biomimetic oxidations catalyzed by transition metal complexes. Imperial College Press, London, pp 461–508
Fridovich I (1995) Ann Rev Biochem 64:97–112
Miller AF, Sorkin DL (1997) Comments Mol Cell Biophys 9(1):1–48
Touati D (1997) In: Scandalios JG (eds) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Plainview, pp 447–493
Bannister JV, Bannister WH, Rotilio G (1987) CRC Crit Rev Biochem 22(2):111–180
Fridovich I (1975) Ann Rev Biochem 44:147–159
Kim EJ, Chung HJ, Suh B, Hah YC, Roe JH (1998) Mol Microbiol 27(1):187–195
Lee J-W, Roe J-H, Kang SO (2002) Methods Enzymol 349:90–101
Uudsemaa M, Tamm T (2003) J Phys Chem A 107(46):9997–10003
Fee JA, Valentine JS (1977) In: Michelson AM, McCord JM, Fridovich I (eds) Superoxide and superoxide dismutases. Academic Press, London, pp 25–28
Bordo D, Matak D, Djinovic-Carugo K, Rosano C, Pesce A, Bolognesi M, Stroppolo ME, Falconi M, Battistoni A, Desideri A (1999) J Mol Biol 285(1):283–296
Borgstahl GE, Parge HE, Hickey MJ, Beyer WF Jr, Hallewell RA, Tainer JA (1992) Cell 71(1):107–118
Lah MS, Dixon MM, Pattridge KA, Stallings WC, Fee JA, Ludwig ML (1995) Biochemistry 34(5):1646–1660
Tierney DL, Fee JA, Ludwig ML, Pennerhahn JE (1995) Biochemistry 34(5):1661–1668
Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA, Getzoff ED (2004) Biochemistry 43(25):8038–8047
Wuerges J, Lee JW, Yim YI, Yim HS, Kang SO, Carugo KD (2004) Proc Natl Acad Sci USA 101(23):8569–8574
Darnault C, Volbeda A, Kim EJ, Legrand P, Vernede X, Lindahl PA, Fontecilla-Camps JC (2003) Nat Struct Biol 10(4):271–279
Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL (2002) Science 298(5593):567–572
Dobbek H, Svetlitchnyi V, Gremer L, Huber R, Meyer O (2001) Science 293(5533):1281–1285
Volbeda A, Charon MH, Piras C, Hatchikian EC, Frey M, Fontecilla-Camps JC (1995) Nature 373(6515):580–587
Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK (1997) Science 278(5342):1457–1462
Jabri E, Carr MB, Hausinger RP, Karplus PA (1995) Science 268(5213):998–1004
He MM, Clugston SL, Honek JF, Matthews BW (2000) Biochemistry 39(30):8719–8727
Al-Mjeni F, Ju T, Pochapsky TC, Maroney MJ (2002) Biochemistry 41(21):6761–6769
Pochapsky TC, Pochapsky SS, Ju T, Mo H, Al-Mjeni F, Maroney MJ (2002) Nat Struct Biol 9(12):966–972
Fiedler AT, Bryngelson PA, Maroney MJ, Brunold TC (2005) J Am Chem Soc 127(15):5449–5462
Pelmenschikov V, Siegbahn PEM (2006) J Am Chem Soc 128(23):7466–7475
Prabhakar R, Morokuma K, Musaev DG (2006) J Comput Chem 27(12):1438–1445
Jackson TA, Brunold TC (2004) Acc Chem Res 37(7):461–470
Miller AF (2008) Acc Chem Res 41(4):501–510
Rulisek L, Jensen KP, Lundgren K, Ryde U (2006) J Comput Chem 27(12):1398–1414
Vance CK, Miller AF (1998) J Am Chem Soc 120(3):461–467
Carrasco R, Morgenstern-Badarau I, Cano J (2007) Inorg Chim Acta 360(1):91–101
Miller AF, Padmakumar K, Sorkin DL, Karapetian A, Vance CK (2003) J Inorg Biochem 93(1–2):71–83
Herbst RW, Guce A, Bryngelson PA, Higgins KA, Ryan KC, Cabelli DE, Garman SC, Maroney MJ (2009) Biochemistry 48(15):3354–3369
Fisher CL, Cabelli DE, Hallewell RA, Beroza P, Lo TP, Getzoff ED, Tainer JA (1997) Proteins Struct Funct Bioinf 29(1):103–112
Miller AF, Sorkin DL, Padmakumar K (2005) Biochemistry 44(16):5969–5981
Tabares LC, Cortez N, Un S (2007) Biochemistry 46(32):9320–9327
Allan CB, Davidson G, Choudhury SB, Gu ZJ, Bose K, Day RO, Maroney MJ (1998) Inorg Chem 37(17):4166–4167
Chohan BS, Shoner SC, Kovacs JA, Maroney MJ (2004) Inorg Chem 43(24):7726–7734
Grapperhaus CA, Darensbourg MY (1998) Acc Chem Res 31(8):451–459
Shoner SC, Olmstead MM, Kovacs JA (1994) Inorg Chem 33(1):7–8
Bryngelson PA, Arobo SE, Pinkham JL, Cabelli DE, Maroney MJ (2004) J Am Chem Soc 126(2):460–461
Leitch S, Bradley MJ, Rowe JL, Chivers PT, Maroney MJ (2007) J Am Chem Soc 129(16):5085–5095
Padden KM, Krebs JF, MacBeth CE, Scarrow RC, Borovik AS (2001) J Am Chem Soc 123(6):1072–1079
Webb SM (2005) Phys Scr T 115:1011–1014
Ankudinov AL, Ravel B, Rehr JJ, Conradson SD (1998) Phys Rev B 58(12):7565–7576
Johnson OE, Ryan KC, Maroney MJ, Brunold TC (2009) J Biol Inorg Chem
Colpas GJ, Maroney MJ, Bagyinka C, Kumar M, Willis WS, Suib SL, Baidya N, Mascharak PK (1991) Inorg Chem 30(5):920–928
Bielski BH, Cabelli DE (1991) Int J Radiat Biol 59(2):291–319
Fridovich I (1998) J Exp Biol 201(8):1203–1209
Choudhury SB, Lee JW, Davidson G, Yim YI, Bose K, Sharma ML, Kang SO, Cabelli DE, Maroney MJ (1999) Biochemistry 38(12):3744–3752
Cowan JA (1997) Inorganic biochemistry: an introduction, 2nd edn. Wiley-VCH, New York
Szilagyi RK, Bryngelson PA, Maroney MJ, Hedman B, Hodgson KO, Solomon EI (2004) J Am Chem Soc 126(10):3018–3019
Ray M, Hammes BS, Yap GPA, Rheingold AL, Borovik AS (1998) Inorg Chem 37(7):1527–1532
Melnik M, Sramko T, Dunajjurco M, Sirota A, Jona E, Holloway CE (1994) Rev Inorg Chem 14(1–4):1–300
Rosenfield SG, Armstrong WH, Mascharak PK (1986) Inorg Chem 25(17):3014–3018
Baidya N, Olmstead M, Mascharak PK (1991) Inorg Chem 30(5):929–937
Marganian CA, Vazir H, Baidya N, Olmstead MM, Mascharak PK (1995) J Am Chem Soc 117(5):1584–1594
Rosenfield SG, Berends HP, Gelmini L, Stephan DW, Mascharak PK (1987) Inorg Chem 26(17):2792–2797
Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT (2008) J Am Chem Soc 130(24):7592–7606
Calatayud ML, Castro I, Sletten J, Cano J, Lloret F, Faus J, Julve M, Seitz G, Mann K (1996) Inorg Chem 35(10):2858–2865
Halonen P, Tammenkoski M, Niiranen L, Huopalahti S, Parfenyev AN, Goldman A, Baykov A, Lahti R (2005) Biochemistry 44(10):4004–4010
Niemoth-Anderson JD, Rodriguez JA, Lee J-W, Roe J, Yim YI, Cabelli DE, Valentine JS, Kang S-O, Maroney MJ (2000) Book of abstracts, 219th ACS national meeting, San Francisco, CA, INOR-515
Youn HD, Kim EJ, Roe JH, Hah YC, Kang SO (1996) Biochem J 318:889–896
Acknowledgments
This work was supported by grants from the National Science Foundation (CHE-0809188 to M.J.M) and the National Institutes of Health (GM 64631 to T.C.B.) and by a National Institutes of Health Chemistry–Biology Interface Training Grant (T32 GM008505 to O.E.J.). The US Department of Energy, Division of Materials Sciences and Division of Chemical Sciences, supported XAS data collection at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory. The National Institutes of Health supports beamline X3B (formerly X9B) at NSLS. Pulse radiolysis studies were carried out at the Center for Radiation Chemical Research, which is funded under contract DE-AC02-98CH10886 with the US Department of Energy. The authors also acknowledge Peter A. Bryngelson for contributing information regarding wild-type NiSOD and for help in mutagenesis, and Robert W. Herbst for assistance in EPR and ESI-MS data collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ryan, K.C., Johnson, O.E., Cabelli, D.E. et al. Nickel superoxide dismutase: structural and functional roles of Cys2 and Cys6. J Biol Inorg Chem 15, 795–807 (2010). https://doi.org/10.1007/s00775-010-0645-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-010-0645-y