Abstract
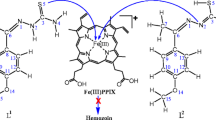
A new class of copper(II) nanohybrid solids, LCu(CH3COO)2 and LCuCl2, have been synthesized and characterized by transmission electron microscopy, dynamic light scattering, and IR spectroscopy, and have been found to be capped by a bis(benzimidazole) diamide ligand (L). The particle sizes of these nanohybrid solids were found to be in the ranges 5–10 and 60–70 nm, respectively. These nanohybrid solids were evaluated for their in vitro antimalarial activity against a chloroquine-sensitive isolate of Plasmodium falciparum (MRC 2). The interactions between these nanohybrid solids and plasmepsin II (an aspartic protease and a plausible novel target for antimalarial drug development), which is believed to be essential for hemoglobin degradation by the parasite, have been assayed by UV–vis spectroscopy and inhibition kinetics using Lineweaver–Burk plots. Our results suggest that these two compounds have antimalarial activities, and the IC50 values (0.025–0.032 μg/ml) are similar to the IC50 value of the standard drug chloroquine used in the bioassay. Lineweaver–Burk plots for inhibition of plasmepsin II by LCu(CH3COO)2 and LCuCl2 show that the inhibition is competitive with respect to the substrate. The inhibition constants of LCu(CH3COO)2 and LCuCl2 were found to be 10 and 13 μM, respectively. The IC50 values for inhibition of plasmepsin II by LCu(CH3COO)2 and LCuCl2 were found to be 14 and 17 μM, respectively. Copper(II) metal capped by a benzimidazole group, which resembles the histidine group of copper proteins (galactose oxidase, β-hydroxylase), could provide a suitable anchoring site on the nanosurface and thus could be useful for inhibition of target enzymes via binding to the S1/S3 pocket of the enzyme hydrophobically. Both copper(II) nanohybrid solids were found to be nontoxic against human hepatocellular carcinoma cells and were highly selective for plasmepsin II versus human cathepsin D. The pivotal mechanism of antimalarial activity of these compounds via plasmepsin II inhibition in the P. falciparum malaria parasite is demonstrated.









Similar content being viewed by others
References
World Health Organization (2008) World Health Organization malaria reports. World Health Organization, Geneva
Greenwood B, Mutabingwa T (2002) Nature 415:670–672
White NJ (1998) Br Med Bull 54:703–715
Ollliaro P, Cattani J, Wirth D (1996) J Am Med Assoc 275(3):230–233
Lew VL, Macdonald L, Ginsburg H, Krugliak M, Tiffert T (2004) Blood Cells Mol Dis 280:353–359
Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE (2002) Proc Natl Acad Sci USA 99:990–995
Roenthal PJ, Sijwali PS, Singh A, Shenai BR (2002) Curr Pharm Des 8:1659–1672
Eggleson KK, Duffin KL, Goldberg DE (1999) J Biol Chem 274:32411–32417
Rosenthal PJ (1998) Emerg Infect Dis 4:49–57
Silva AM, Lee AY, Gulnik SV, Majer P, Collins J, Bhat TN, Collins PJ, Cachau RE, Luker KE, Gluzman IY, Francis SE, Oksman A, Goldberg DE, Erickson JW (1996) Proc Natl Acad Sci USA 93:10034–10039
Muthas D, Nöteberg D, Sabnis YA, Hamelink E, Vrang L, Samuelsson B, Karlen A, Hallberg A (2005) Bioorg Med Chem 13:5371–5390
Dahlgren A, Kvarnstrom I, Vrang L, Hamelink E, Hallberg A, Rosenquist A, Samuelson B (2003) Bioorg Med Chem 11:3423–3437
Nezami A, Luque I, Kimura T, Kiso Y, Ernesto F (2002) Biochemistry 41:2273–2280
Ersmark K, Feierberg I, Bjelic S, Hamelink E, Hackett F, Blackman MJ, Hulten J, Samuelsson B, Aqvist J, Hallberg A (2004) J Med Chem 47:110–122
Ocheskey JA, Polyakov VR, Harpstrite SE, Oksman A, Goldberg DE, Worms DP, Sharma V (2003) J Inorg Biochem 93:265–270
Mondhindru A, Fisher JM, Rabinowitz M (1983) Biochem Pharmacol 32:3627–3632
Gokhale NH, Shirisha KJ, Padhey SB, Croft SL, Kendrick HD, Mckee V (2006) Bioorg Med Chem Lett 16:430–432
Gokhale NH, Padhye SB, Billington DC, Rathbone DL, Croft SL, Kendrick HD, Anson CE, Powell AK (2003) Inorg Chim Acta 349:23–29
Cescon LA, Day AR (1962) J Org Chem 27:581–586
Singla M, Gupta M, Mathur P, Hundal MS (2008) Transition Met Chem 33:175–182
Gupta M, Mathur P, Butcher RJ (2001) Inorg Chem 40:878–885
Luisi PL, Straub BE (1984) Reverse micelles. Plenum Press, New York
Upadhyay SK, Tehlan S, Mathur P (2007) Spectrochim Acta 66:347–352
Ahmad T, Ramanujachary KV, Lofland SE, Ganguli AK (2004) J Mater Chem 14:3406–3410
Jain TK, Roy I, De TK, Maitra A (1998) J Am Chem Soc 120:11092–11095
Trager W, Jensen JB (1976) Science 193:673–675
Agli MD, Parapini S, Galli G, Vaiana N, Taramelli D, Sparatore A, Liu P, Dunn BM, Bosisio E, Romeo S (2006) J Med Chem 49:7440–7449
Lowry OH, Rosbrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Goldberg DE, Slater AFG, Beavis R, Chait B, Cerami A, Henderson GB (1991) J Exp Med 173:961–969
Hill J, Tyas L, Phylip LH, Kay J, Dunn BM, Berry C (1994) FEBS Lett 352:155–158
Copeland RA (ed) (1996) Enzymes: a practical introduction to structure, mechanism and data analysis. Wiley, New York
Mosmann T (1983) J Immunol Methods 65:55–63
Ren H, Grady S, Gammenara D, Heinzen H, Moyna P, Croft S, Kendrick H, Yardley V, Moyna G (2001) Bioorg Med Chem Lett 11:1851–1854
Jiang S, Prigge ST, Wei L, Gao YE, Hudson TH, Gerena L, Dame JB, Kyle DE (2001) Antimicrob Agents Chemother 45(9):2577–2584
Muegge I, Martin Y (1999) J Med Chem 42:791–804
Rosenthal PJ (1995) Exp Parasitol 80:272–281
Dluzewski AR, Rangachari K, Wilson RJM, Gratzer WB (1986) Exp Parasitol 62:416–422
Rosenthal PJ, McKerrow JH, Aikawa M, Nagasawa H, Leech JH (1988) J Clin Invest 82:1560–1566
Vander Jagt DL, Caughey WS, Campos NM, Hunsaker LA, Zanner MA (1989) Prog Clin Biol Res 313:105–118
Bailly E, Jambou R, Savel J, Jaureguiberry G (1992) J Protozool 39:593–599
Asawamahasakda W, Ittarat I, Chang C-C, McElroy P, Meshnick SR (1994) Mol Biochem Parasitol 67:183–191
Tehlan S, Hundal MS, Mathur P (2004) Inorg Chem 43:6589–6595
Sharma A, Eapen A, Subbarao SK (2005) J Biol Chem Tokyo 138:71–78
Acknowledgments
The authors are thankful to Benn M. Dunn (Department of Biochemistry and Molecular Biology, College of Medicine, University of Florida, Gainesville, FL, USA) for helpful discussion and donating the plasmid for proplasmepsin II. We are also thankful to Vineeta Singh and C.R. Pillai, Parasite Bank at NIMR, for help in performing red blood cell parasite inhibition assays. Thanks are also due to Bhanu Arya, Technical Officer, and Poonam Gupta, Technical Assistant, for excellent technical help during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohapatra, S.C., Tiwari, H.K., Singla, M. et al. Antimalarial evaluation of copper(II) nanohybrid solids: inhibition of plasmepsin II, a hemoglobin-degrading malarial aspartic protease from Plasmodium falciparum . J Biol Inorg Chem 15, 373–385 (2010). https://doi.org/10.1007/s00775-009-0610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0610-9