Abstract
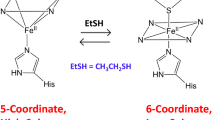
To evaluate the potential of using heme-containing lipocalin nitrophorin 1 (NP1) as a template for protein engineering, we have replaced the native axial heme-coordinating histidine residue with glycine, alanine, and cysteine. We report here the characterization of the cysteine mutant H60C_NP1 by spectroscopic and crystallographic methods. The UV/vis, resonance Raman, and magnetic circular dichroism spectra suggest weak thiolate coordination of the ferric heme in the H60C_NP1 mutant. Reduction to the ferrous state resulted in loss of cysteine coordination, while addition of exogenous imidazole ligands gave coordination changes that varied with the ligand. Depending on the substitution of the imidazole, we could distinguish three heme coordination states: five-coordinate monoimidazole, six-coordinate bisimidazole, and six-coordinate imidazole/thiolate. Ligand binding affinities were measured and found to be generally 2–3 orders of magnitude lower for the H60C mutant relative to NP1. Two crystal structures of the H60C_NP1 in complex with imidazole and histamine were solved to 1.7- and 1.96-Å resolution, respectively. Both structures show that the H60C mutation is well tolerated by the protein scaffold and suggest that heme–thiolate coordination in H60C_NP1 requires some movement of the heme within its binding cavity. This adjustment may be responsible for the ease with which the engineered heme–thiolate coordination can be displaced by exogenous ligands.











Similar content being viewed by others
Abbreviations
- BuImd:
-
N-(n-Butyl)-imidazole
- H60C_NP1:
-
Nitrophorin 1 mutant in which the axial His-60 ligand is replaced by Cys-60
- MCD:
-
Magnetic circular dichroism
- NP1:
-
Nitrophorin 1
- NP4:
-
Nitrophorin 4
- PBS:
-
Phosphate-buffered saline
- RMSD:
-
Root mean square deviation
- RR:
-
Resonance Raman
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Armstrong FA, Bond AM, Buchi FN, Hamnett A, Hill HA, Lannon AM, Lettington OC, Zoski CG (1993) Analyst 118(8):973–978
Iffland A, Tafelmeyer P, Saudan C, Johnsson K (2000) Biochemistry 39(35):10790–10798
Musah RA, Goodin DB (1997) Biochemistry 36(39):11665–11674
Musah RA, Jensen GM, Bunte SW, Rosenfeld RJ, Goodin DB (2002) J Mol Biol 315:845–857
Hirst J, Goodin DB (2000) J Biol Chem 275:8582–8591
Erman JE, Vitello LB, Miller MA, Kraut J (1992) J Am Chem Soc 114(16):6592–6593
Joo H, Lin Z, Arnold FH (1999) Nature 399(17):670–673
Cirino PC, Arnold FH (2002) Curr Opin Chem Biol 6(2):130–135
Glieder A, Farinas ET, Arnold FH (2002) Nat Biotechnol 20(11):1135–1139
May O, Voigt CA, Arnold FH (2002) Enzyme catalysis in organic synthesis, vol 1, 2nd edn. Wiley-VCH, Weinheim, Germany, pp 95–138
Cirino PC, Arnold FH (2003) Angew Chem Int Ed 42(28):3299–3301
Peters MW, Meinhold P, Glieder A, Arnold FH (2003) J Am Chem Soc 125:13442–13450
Farinas ET, Alcalde M, Arnold FH (2004) Tetrahedron 60:525–528
Ozaki S-I, Roach MP, Matsui T, Watanabe Y (2001) Acc Chem Res 34:818–825
Sigman JA, Kwok BC, Lu Y (2000) J Am Chem Soc 122(34):8192–8196
Allocatelli CT, Cutruzzola F, Brancaccio A, Vallone B, Brunori M (1994) FEBS Lett 356(1):151
Ikeda-Saito M, Hori H, Andersson LA, Prince RC, Pickering IJ, George GN, Sanders CD, Lutz RS, McKelvey EJ, Mattera R (1992) J Biol Chem 267(32):22843–22852
Sono M, Roach MP, Coulter ED, Dawson JH (1996) Chem Rev 96:2841–2887
Atkins WM, Sligar SG (1989) J Am Chem Soc 111:2715–2717
Poulos TL (1996) J Biol Inorg Chem 1:356–359
Goodin DB, McRee DE (1993) Biochemistry 32:3313–3324
Watanabe Y, Groves JT (1992) Enzymes, vol 20, 3rd edn, Academic, San Diego, pp 405–452
Dawson JH (1988) Science 240(4851):433–439
Green MT, Dawson JH, Gray HB (2004) Science 304:1653–1656
Adachi S, Nagano S, Ishimori K, Watanabe Y, Morishima I, Egawa T, Kitagawa T, Makino R (1993) Biochemistry 32(1):241–252
Liu Y, Moeenne-Loccoz P, Hildebrand DP, Wilks A, Loehr TM, Mauk AG, Ortiz de Montellano PR (1999) Biochemistry 38(12):3733–3743
Sigman JA, Pond AE, Dawson JH, Lu Y (1999) Biochemistry 38(34):11122–11129
Hildebrand DP, Ferrer JC, Tang H-L, Smith M, Mauk AG (1995) Biochemistry 34:11598–11605
Wang W-H, Lu J-X, Yao P, Xie Y, Huang Z-X (2003) Protein Eng 16(12):1047–1054
Perera R, Sono M, Sigman JA, Pfister TD, Lu Y, Dawson JH (2003) Proc Natl Acad Sci USA 100(7):3641–3646
Ribeiro JMC, Hazzard JMH, Nussenzveig RH, Champagne DE, Walker AF (1993) Science 260(23):539–541
Oliveira PL, Kawooya JK, Ribeiro JMC, Meyer T, Poorman R, Alves EW, Walker FA, Machado EA, Nussenzveig RH, Padovan GJ, Masuda H (1995) J Biol Chem 270(18):10897–10891
Montfort WR, Weichsel A, Andersen JF (2000) Biochim Biophys Acta 1482:110–118
Flower DR, North AC, Attwood TK (1993) Protein Sci 2:753–761
Flower DR, North ACT, Sansom CE (2000) Biochim Biophys Acta 1482:9–24
Weichsel A, Andersen JF, Champagne DE, Walker FA, Montfort WR (1998) Nat Struct Biol 5(4):304–309
Andersen JF, Champagne DE, Weichsel A, Ribeiro JMC, Bulfour CA, Dress V, Montfort WR (1997) Biochemistry 36:4423–4428
Akoyunoglou J-HA, Olcott HS, Brown WD (1963) Biochemistry 2(5):1033–1041
Kriss GA (1994) In: Crabtree DR, Hanisch RJ, Barnes J (eds) Astronomical data analysis software & systems III. Astronomical Society of the Pacific, San Francisco
Huff AM, Chang CK, Cooper DK, Smith KM, Dawson JH (1993) Inorg Chem 32:1460–1466
Collaborative Computational Project, N (1994) Acta Crystallogr D 50:760–763
Pflugrath JW (1999) Acta Crystallogr D 55:1718–1725
Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Acta Crystallogr D 54:905–921
Bailey S (1994) Acta Crystallogr D 50:760–763
McLachlan AD (1982) Acta Crystallogr A 38:871–873
Hutchinson EG, Thornton JM (1996) Protein Sci 5:212–220
Ribeiro JMC, Walker FA (1994) J Exp Med 180:2251–2257
Sono M, Andersson LA, Dawson JH (1982) J Biol Chem 257(14):8308–8320
Psylinakis E, Davoras EM, Ioannidis N, Trikeriotis M, Petrouleas V, Ghanotakis DF (2001) Biochim Biophys Acta 1533:119–127
Hirst J, Wilcox SK, Ai J, Moënne-Loccoz P, Loehr TM, Goodin DB (2001) Biochemistry 40(5):1274–1283
Hirst J, Wilcox SK, Williams PA, Blankenship J, McRee DE, Goodin DB (2001) Biochemistry 40(5):1265–1273
Sono M, Stuehr DJ, Ikeda-Saito M, Dawson JH (1995) J Biol Chem 270(34):1993–19948
Andersson L, Mylrajan M, Sullivan E Jr, Strauss S (1989) J Biol Chem 164:19099–19102
Hu S, Morris IK, Singh JP, Smith KM, Spiro TG (1993) J Am Chem Soc 115:12446–12458
Hu S, Smith KM, Spiro TG (1996) J Am Chem Soc 118:12638–12646
Lou BS, Snyder JK, Marshall P, Wang JS, Wu G, Kulmacz RJ, Tsai AL, Wang J (2000) Biochemistry 39(40):12424–12434
Spiro TG, Li XY (eds) (1987) Biological applications of Raman spectroscopy. Wiley, New York
Spiro TG, Stong JD, Stein P (1979) J Am Chem Soc 101:2648–2655
Wang J, Caughey WS, Rousseau DL (1996) In: Feelisch M, Stamler J (eds) Methods in nitric oxide research. Wiley, New York
Maes EM, Walker FA, Montfort WR, Czernuszewicz RS (2001) J Am Chem Soc 123(47):11664–11672
Sun J, Loehr TM, Wilks A, Ortiz de Montellano PR (1994) Biochemistry 33(46):13734–13740
Anzenbacher P, Evangelista-Kirkup R, Schenkman J, Spiro TG (1989) Inorg Chem 28(25):4491–4495
Vogel KM, Kozlowski PM, Zgierski MZ, Spiro TG (2000) Inorg Chim Acta 297(1–2):11–17
Spiro TG, Kozlowski P (2001) Acc Chem Res 34:137–144
Li T, Quillin ML, Phillips GN, Olson JS (1994) Biochemistry 33:1433–1446
Feis A, Rodriguez-Lopez JN, Thorneley RNF, Smulevich G (1998) Biochemistry 37:13575–13581
Terentis AC, Thomas SR, Takikawa O, Littlejohn TK, Truscott RJW, Armstrong RS, Yeh S-R, Stocker R (2002) J Biol Chem 277:15788–15794
Weichsel A, Andersen JF, Roberts SA, Montfort WR (2000) Nat Struct Biol 7(7):551–554
Maes EM, Weichsel A, Anderson JF, Shepley D, Montfort WR (2004) Biochemistry 43:6679–6690
Shokhireva TK, Shokhirev NV, Walker FA (2003) Biochemistry 42:679–693
Roberts SA, Weichsel A, Qiu Y, Shelnutt JA, Walker FA, Montfort WR (2001) J Biol Chem 40(38):11327–11337
Andersen JF, Ding XD, Balfour C, Shokhireva TK, Champagne DE, Walker AF, Montfort WR (2000) Biochemistry 39:10118–10131
Nienhaus K, Maes EM, Weichsel A, Montfort WR, Nienhaus GU (2004) J Biol Chem 279(38):39401–39407
Acknowledgments
The plasmid for the NP1 gene was provided by F.A. Walker, who we thank for a number of helpful discussions. We thank C.D. Stout for advice and help during X-ray data acquisition and structure refinement. We also thank David Ginsberg for assistance during protein expression and purification. This work was supported by National Institute of Health Grants GM41049 (to D.B.G.) and GM26730 (to J.H.D.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vetter, S.W., Terentis, A.C., Osborne, R.L. et al. Replacement of the axial histidine heme ligand with cysteine in nitrophorin 1: spectroscopic and crystallographic characterization. J Biol Inorg Chem 14, 179–191 (2009). https://doi.org/10.1007/s00775-008-0436-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-008-0436-x