Abstract
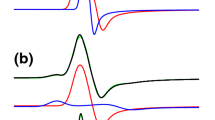
Methyl-coenzyme M reductase (MCR) catalyzes the formation of methane from methyl-coenzyme M and coenzyme B in methanogenic archaea. The enzyme has two structurally interlinked active sites embedded in an α2β2γ2 subunit structure. Each active site has the nickel porphyrinoid F430 as a prosthetic group. In the active state, F430 contains the transition metal in the Ni(I) oxidation state. The active enzyme exhibits an axial Ni(I)-based continuous wave (CW) electron paramagnetic resonance (EPR) signal, called red1a in the absence of substrates or red1c in the presence of coenzyme M. Addition of coenzyme B to the MCR-red1 state can partially and reversibly convert it into the MCR-red2 form, which shows a rhombic Ni(I)-based EPR signal (at X-band microwave frequencies of approximately 9.4 GHz). In this report we present evidence from high-field/high-frequency CW EPR spectroscopy (W-band, microwave frequency of approximately 94 GHz) that the red2 state consists of two substates that could not be resolved by EPR spectroscopy at X-band frequencies. At W-band it becomes apparent that upon addition of coenzyme B to MCR in the red1c state, two red2 EPR signals are induced, not one as was previously believed. The first signal is the well-characterized (ortho)rhombic EPR signal, thus far called red2, while the second previously unidentified signal is axial. We have named the two substates MCR-red2r and MCR-red2a after their rhombic and axial signals, respectively.




Similar content being viewed by others
Abbreviations
- CH3-S-CoM:
-
Methyl-coenzyme M
- CW:
-
Continuous wave
- EPR:
-
Electron paramagnetic resonance
- HS-CoB:
-
Coenzyme B
- HS-CoM:
-
Coenzyme M
- HYSCORE:
-
Hyperfine sublevel correlation
- MCR:
-
Methyl-coenzyme M reductase
References
Thauer RK (1998) Microbiology 144:2377–2406
Thauer RK, Jungermann K, Decker K (1977) Bacteriol Rev 41:100–180
Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK (1997) Science 278:1457–1462
Goubeaud M, Schreiner G, Thauer RK (1997) Eur J Biochem 243:110–114
Pelmenschikov V, Blomberg MRA, Siegbahn PEM, Crabtree RH (2002) J Am Chem Soc 124:4039
Kunz RC, Horng YC, Ragsdale SW (2006) J Biol Chem 281:34663–34676
Mahlert F, Grabarse W, Kahnt J, Thauer RK, Duin EC (2002) J Biol Inorg Chem 7:101–112
Goenrich M, Duin EC, Mahlert F, Thauer RK (2005) J Biol Inorg Chem 10:333–342
Wasserfallen A, Nolling J, Pfister P, Reeve J, Conway de Macario E (2000) Int J Syst Evol Microbiol 50(Pt 1):43–53
Gunsalus RP, Romesser JA, Wolfe RS (1978) Biochemistry 17:2374–2377
Kobelt A, Pfaltz A, Ankel-Fuchs D, Thauer RK (1987) FEBS Lett 214:265–268
Ellermann J, Hedderich R, Böcher R, Thauer RK (1988) Eur J Biochem 172:669–677
Rospert S, Linder D, Ellermann J, Thauer RK (1990) Eur J Biochem 194:871–877
Bonacker LG, Baudner S, Mörschel E, Böcher R, Thauer RK (1993) Eur J Biochem 217:587–595
Duin EC, Signor L, Piskorski R, Mahlert F, Clay MD, Goenrich M, Thauer RK, Jaun B, Johnson MK (2004) J Biol Inorg Chem 9:563–576
Mahlert F, Bauer C, Jaun B, Thauer RK, Duin EC (2002) J Biol Inorg Chem 7:500–513
Bradford MM (1976) Anal Biochem 72:248–254
Stoll S, Schweiger A (2006) J Magn Reson 178:42
Finazzo C, Harmer J, Bauer C, Jaun B, Duin EC, Mahlert F, Goenrich M, Thauer RK, Van Doorslaer S, Schweiger A (2003) J Am Chem Soc 125:4988–4989
Finazzo C, Harmer J, Jaun B, Duin EC, Mahlert F, Thauer RK, Van Doorslaer S, Schweiger A (2003) J Biol Inorg Chem 8:586–593
Acknowledgements
We thank the Swiss National Science Foundation (SNF) and the Fonds der Chemischen Industrie for financial support. D.H. gratefully acknowledges a research scholarship (HI 1094/1-1) from the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kern, D.I., Goenrich, M., Jaun, B. et al. Two sub-states of the red2 state of methyl-coenzyme M reductase revealed by high-field EPR spectroscopy. J Biol Inorg Chem 12, 1097–1105 (2007). https://doi.org/10.1007/s00775-007-0281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0281-3