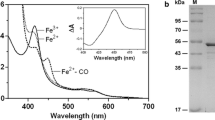
Abstract
This work provides functional data showing that the bacterial CYP102A1 recognises compounds metabolised by human CYP3A4, CYP2E1 and CYP1A2 and is able to catalyse different reactions. Wild-type cytochrome CYP102A1 from Bacillus megaterium is a catalytically self-sufficient enzyme, containing an NADPH-dependent reductase and a P450 haem domain fused in a single polypeptidie chain. An NADPH-dependent method (Tsotsou et al. in Biosens. Bioelectron. 17:119–131, 2002) together with spectroscopic assays were applied to investigate the catalytic activity of CYP102A1 towards 19 xenobiotics, including 17 commercial drugs. These molecules were chosen to represent typical substrates of the five main families of drug-metabolising human cytochromes P450. Liquid chromatography–mass spectrometry analysis showed that CYP102A1 catalyses the hydroxylation of chlorzoxazone, aniline and p-nitrophenol, as well as the N-dealkylation of propranolol and the dehydrogenation of nifedipine. These drugs are typical substrates of human CYP2E1 and CYP3A4. The K M values calculated for these compounds were in the millimolar range: 1.21 ± 0.07 mM for chlorzoxazone, 2.52 ± 0.08 mM for aniline, 0.81 ± 0.04 mM for propranolol. The values of v max for chlorzoxazone and propranolol were 46.0 ± 9.0 and 7.6 ± 3.4 nmol min−1 nmol−1, respectively. These values are higher then those measured for the human enzymes. The v max value for aniline was 9.4 ± 1.3 nmol min−1 nmol−1, comparable to that calculated for human cytochromes P450. The functional data were found to be in line with the sequence alignments, showing that the identity percentage of CYP102A1 with CYP3A4 and CYP2E1 is higher than that found for CYP1A2, CYP2C9 and CYP2D6 families.





Similar content being viewed by others
References
Guengerich FP (2003) Mol Interv 3:194–204
Ortiz de Montellano PR, De Voss JJ (2002) Nat Prod Rep 19:477–493
Guengerich FP (2001) Curr Drug Metab 2:93–115
Luo G, Guenthner T, Gan LS, Humphreys WG (2004) Curr Drug Metab 5:483–505
Narhi LO, Fulco AJJ (1986) Biol Chem 261:7160–7169
Ravichandran KG, Boddupalli SS, Hasermann CA, Peterson JA, Deisenhofer J (1993) Science 261:731–736
Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE (2000) Mol Cell 5:121–131
Munro AW, Leys DG, McLean KJ, Marshall KR, Ost TW, Daff S, Miles CS, Chapman SK, Lysek DA, Moser CC, Page CC, Dutton PL (2002) Trends Biochem Sci 27:250–257
Noble MA, Miles CS, Chapman SK, Lysek DA, MacKay AC, Reid GA, Hanzlik RP, Munro AW (1999) Biochem J 339:371–379
Munro AW, Daff S, Coggins JR, Lindsay JG, Chapman SK (1996) Eur J Biochem 239:403–409
Miura Y, Fulco AJ (1975) Biochim Biophys Acta 388:305–317
Ost TW, Miles CS, Murdoch J, Cheung Y, Reid GA, Chapman SK, Munro AW (2000) FEBS Lett 486:173–177
Seng Wong T, Arnold FH, Schwaneberg U (2004) Biotechnol Bioeng 85:351–358
Meinhold P, Peters MW, Chen MM, Takahashi K, Arnold FH (2005) Chembiochem 6:1765–1768
Lussenburg BM, Babel LC, Vermeulen NP, Commandeur JN (2005) Anal Biochem 341:148–155
Otey CR, Bandara G, Lalonde J, Takahashi K, Arnold FH (2005) Biotechnol Bioeng 93:494–499
Tsotsou GE, Cass AE, Gilardi G (2002) Biosens Bioelectron 17:119–131
Li H, Poulos TL (1997) Nat Struct Biol 4:1400–1406
Fairhead MJ, Giannini S, Gillam EMJ, Gilardi G (2005) J Biol Inorg Chem 10:842–10853
Werringloer J (1978) Methods Enzymol 52:297–302
Semple HA, Xia F (1994) J Chromatogr B 655:293–299
Streel B, Zimmer C, Sibenaler R, Ceccato A (1998) J Chromatogr B 720:119–128
Wang RW, Newton DJ, Scheri TD, Lu AY (1997) Drug Metab Dispos 25:502–507
Patki KC, Von Moltke LL, Greenblatt DJ (2003) Drug Metab Dispos 31:938–944
Narimatsu S, Kobayashi N, Masubuchi Y, Horie T, Kakegawa T, Kobayashi H, Hardwick JP, Gonzalez FJ, Shimada N, Ohmori S, Kitada M, Asaoka K, Kataoka H, Yamamoto S, Satoh T (2000) Chem Biol Interact 127:73–90
Gillam EM, Guo Z, Guengerich FP (1994) Arch Biochem Biophys 312:59–66
Yamazaki H, Nakano M, Gillam EM, Bell LC, Guengerich FP, Shimada T (1996) Biochem Pharmacol 52:301–309
Zhang TY, Zhu YX, Gunaratna C (2002) J Chromatogr B 780:371–379
Loida PJ, Sligar SG (1993) Biochemistry 32:11530–11538
Beaudry F, Yves Le Blanc JC, Coutu M, Ramier I, Moreau JP, Brown NK (1999) Biomed Chromatogr 13:363–369
Zerilli A, Ratanasavanh D, Lucas D, Goasduff T, Dreano Y, Menard C, Picart D, Berthou F (1997) Chem Res Toxicol 10:1205–1212
Nedelcheva V, Gut I, Soucek P, Tichavska B, Tynkova L, Mraz J, Guengerich FP, Ingelman-Sundberg M (1999) Arch Toxicol 73:33–40
McGinnity DF, Parker AJ, Soars M, Riley RJ (2000) Drug Metab Dispos 28:1327–1334
Peter R, Bocker R, Beaune PH, Iwasaki M, Guengerich FP, Yang CS (1990) Chem Res Toxicol 3:566–573
Tassaneeyakul W, Veronese ME, Birkett DJ, Miners JO (1993) J Chromatogr 616:73–78
Gorski JC, Jones DR, Wrighton SA, Hall SD (1997) Xenobiotica 27:243–256
Ono S, Hatanaka T, Hotta H, Tsutsui M, Satoh T, Gonzalez FJ (1995) Pharmacogenetics 5:143–150
Anari MR, Bakhtiar R, Franklin RB, Pearson PG, Bailliem TA (2003) Anal Chem 75:469–478
Guengerich FP, Martin MV, Beaune PH, Kremers P, Wolff T, Waxman DJ (1986) J Biol Chem 261:5051–5060
Lewis DF, Watson E, Lake BG (1998) Mutat Res 410:245–270
Degtyarenko KN, Archakov AI (1993) FEBS Lett 332:1–8
Acknowledgements
G.D.N. and G.G. gratefully acknowledge the ISI foundation for a Lagrange postdoctoral fellowship and Programma di Ricareca di Interesse Nazionale (PRIN) for financial support. Thanks are due to Graham Taylor for help with MS experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Nardo, G., Fantuzzi, A., Sideri, A. et al. Wild-type CYP102A1 as a biocatalyst: turnover of drugs usually metabolised by human liver enzymes. J Biol Inorg Chem 12, 313–323 (2007). https://doi.org/10.1007/s00775-006-0188-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0188-4