Abstract
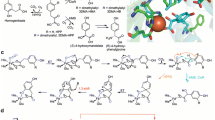
Human porphobilinogen synthase [EC.4.2.1.24] is a homo-octamer enzyme. In the active center of each subunit, four cysteines are titrated with 5,5′-dithiobis(2-nitrobenzoic acid). Cys122, Cys124 and Cys132 are placed near two catalytic sites, Lys199 and Lys252, and coordinate a zinc ion, referred to as “a proximal zinc ion”, and Cys223 is placed at the orifice of the catalytic cavity and coordinates a zinc ion, referred to as “a distal zinc ion”, with His131 . When the wild-type enzymes C122A (Cys122→Ala), C124A (Cys124→Ala), C132A (Cys132→Ala) and C223A (Cys223→Ala) were oxidized by hydrogen peroxide, the levels of activity were decreased. Two cysteines were titrated with 5,5′-dithiobis(2-nitrobenzoic acid) in the wild-type enzyme, while on the other hand, one cysteine was titrated in the mutant enzymes. When wild-type and mutant enzymes were reduced by 2-mercaptoethanol, the levels of activity were increased: four and three cysteines were titrated, respectively, suggesting that a disulfide bond was formed among Cys122, Cys124 and Cys132 under oxidizing conditions. We analyzed the enzyme-bound zinc ion of these enzymes using inductively coupled plasma mass spectrometry with gel-filtration chromatography. The results for C223A showed that the number of proximal zinc ions correlated to the level of enzymatic activity. Furthermore, zinc-ion-free 2-mercaptoethanol increased the activity of the wild-type enzyme without a change in the total number of zinc ions, but C223A was not activated. These findings suggest that a distal zinc ion moved to the proximal binding site when a disulfide bond among Cys122, Cys124 and Cys132 was reduced by reductants. Thus, in the catalytic functioning of the enzyme, the distal zinc ion does not directly contribute but serves rather as a reserve as the next proximal one that catalyzes the enzyme reaction. A redox change of the three cysteines in the active center accommodates the catch and release of the reserve distal zinc ion placed at the orifice of the catalytic cavity.



Similar content being viewed by others
Abbreviations
- δ-ALA:
-
δ-Aminolevulinic acid
- bis-tris:
-
[Bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane
- bp:
-
Base pair
- DEAE:
-
Diethylaminoethyl
- DTNB:
-
5,5′-Dithiobis(2-nitrobenzoic acid)
- EDTA:
-
Ethylenediaminetetraacetic acid
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LB:
-
Luria–Bertani
- 2-ME:
-
2-Mercaptoethanol
- PBGS:
-
Porphobilinogen synthase
- PCR:
-
Polymerase chain reaction
- PMSF:
-
Phenylmethylsulfonylfluoride
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Shemin D, Russell CS (1953) J Am Chem Soc 75:4873–4874
Shemin D (1972) In: Boyer P (ed) The enzymes, vol 7, 3rd edn. Academic Press, New York, pp 323–337
Jaffe EK (1995) J Bioenerg Biomembr 27:169–179
Shoolingin-Jordan PM (1998) Biochem Soc Trans 26:326–336
Breinig S, Kervinen J, Stith L, Wasson AS, Fairman R, Wlodawer A, Zdanov A, Jaffe EK (2003) Nat Struct Biol 10:757–763
Erskine PT, Senior N, Awan S, Lambert R, Lewis G, Tickle IJ, Sarwar M, Spencer P, Thomas P, Warren MJ, Shoolingin-Jordan PM, Wood SP, Cooper JB (1997) Nat Struct Biol 4:1025–1031
Erskine PT, Newbold R, Roper J, Coker A, Warren MJ, Shoolingin-Jordan PM, Wood SP, Cooper JB (1999) Protein Sci 8:1250–1256
Erskine PT, Newbold R, Brindley AA, Wood SP, Shooligin-Jordan PM, Warren MJ, Cooper JB (2001) J Mol Biol 312:133–141
Erskine PT, Duke EM, Tickle IJ, Senior NM, Warren MJ, Cooper JB (2000) Acta Crystallogr D 56:421–430
Kervinen J, Jaffe EK, Stauffer F, Neier R, Wlodawer A, Zdanov A (2001) Biochemistry 40:8227–8236
Erskine PT, Norton E, Cooper JB, Lambert R, Coker A, Lewis G, Spencer P, Sarwar M, Wood SP, Warren MJ, Shoolingin-Jordan PM (1999) Biochemistry 38:4266–4276
Jaffe EK, Kervinen J, Martins J, Stauffer F, Neier R, Wlodawer A, Zdanov A (2002) J Biol Chem 277:19792–19799
Frankenberg N, Erskine PT, Cooper JB, Shoolingin-Jordan PM, Jahan D, Heinz DW (1999) J Mol Biol 289:591–602
Cheh A, Neilands JB (1973) Biochem Biophys Res Commun 55:1060–1063
Bevan DR, Bodlaender P, Shemin D (1980) J Biol Chem 255:2030–2035
Jaffe EK, Salowe SP, Chen NT, DeHaven PA (1984) J Biol Chem 259:5032–5036
Dent AJ, Beyersmann D, Block C, Hasnain SS (1990) Biochemistry 29:7822–7828
Erskine PT, Coates L, Newbold R, Brindley AA, Stauffer F, Wood SP, Warren MJ, Cooper JB, Shoolingin-Jordan PM, Neier R (2001) FEBS Lett 503:196–200
Shoolingin-Jordan PM, Spencer P, Sarwar M, Erskine PE, Cheung KM, Cooper JB, Norton EB (2002) Biochem Soc Trans 30:584–590
Frère F, Schubert W, Stauffer F, Frankenberg N, Neier R, Jahn D, Heinz DW (2002) J Mol Biol 320:237–247
Spencer P, Jordan PM (1993) Biochem J 290:279–287
Tsukamoto I, Yoshinaga T, Sano S (1979) Biochim Biophys Acta 570:167–178
Jaffe EK, Martins J, Li J, Kervinen J, Dunbrack RL Jr (2001) J Biol Chem 276:1531–1537
Kundrat L, Martins J, Stith L, Dunbrack RL Jr, Jaffe EK (2003) J Biol Chem 278:31325–31330
Wetmur JG, Bishop DF, Cantelmo C, Desnick RJ (1986) Proc Natl Acad Sci USA 83:7703–7707
Jaffe EK, Volin M, Bronson-Mullins CR, Dunbrack RL Jr, Kervinen J, Martins J, Quinlan JF Jr, Sazinsky MH, Steinhouse EM, Yeung AT (2000) J Biol Chem 275:2619–2626
Williams CH Jr, Arscott LD, Mattews RG, Thorpe C, Wilkenson KD (1979) Meth Enzymol 62:185–198
Ellman GL (1959) Arch Biochem Biophys 82:70–77
Laemmli UK (1970) Nature 227:680–685
Acknowledgements
We thank Yoko Endo, Occupational Poisoning Center, Tokyo Rosai Hospital, for her helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawada, N., Nagahara, N., Sakai, T. et al. The activation mechanism of human porphobilinogen synthase by 2-mercaptoethanol: intrasubunit transfer of a reserve zinc ion and coordination with three cysteines in the active center. J Biol Inorg Chem 10, 199–207 (2005). https://doi.org/10.1007/s00775-005-0629-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0629-5