Abstract
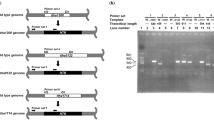
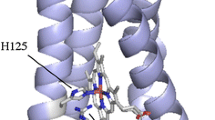
KatB is the only catalase–peroxidase identified so far in Sinorhizobium meliloti. It plays a housekeeping role, as it is expressed throughout all the growth phases of the free-living bacterium and also during symbiosis. This paper describes the functional and structural characterization of the KatB mutants Gly303Ser, Trp95Ala, Trp95Phe, Tyr217Leu, Tyr217Phe and Met243Val carried out by optical and electron spin resonance spectroscopy. The aim of this work was to investigate the involvement of these residues in the catalatic and/or peroxidatic reaction and falls in the frame of the open dispute around the factors that influence the balance between catalatic and peroxidatic activity in heme enzymes. The Gly303 residue is not conserved in any other protein of this family, whereas the Trp95, Tyr217 and Met243 residues are thought to form an intrinsic cofactor that is likely to play a role in intramolecular electron transfer. Spectroscopic investigations show that the Gly303Ser mutant is almost similar to the wild-type KatB and should not be involved in substrate binding. Mutations on Trp95, Tyr217 and Met243 clear out the catalatic activity completely, whereas the peroxidatic activity is maintained or even increased with respect to that of the wild-type enzyme. The k cat values obtained for these mutants suggest that Trp95 and Tyr217 form a huge delocalized system that provides a pathway for electron transfer to the heme. Conversely, Met243 is likely to be placed close to the binding site of the organic molecules and plays a crucial role in substrate docking.







Similar content being viewed by others
Abbreviations
- ABTS:
-
2, 2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- APX:
-
Ascorbate peroxidase
- CCP:
-
Cytochrome c peroxidase
- CT:
-
Charge transfer
- DMAB:
-
3-Dimethylaminobenzoic acid
- ESR:
-
Electron spin resonance
- LPO:
-
Lactoperoxidase
- MBTH:
-
3-Methyl-2-benzothiazolinone hydrazone hydrochloride
- MPO:
-
Myeloperoxidase
- PAA:
-
Peracetic acid
- RZ:
-
Reinheitszahl
References
Klotz MG, Loewen PC (2003) Mol Biol Evol 20:1098–1112
Welinder KG (1991) Biochim Biophys Acta 1080:215–220
Welinder KG, Mauro JM, Norskov-Lauritsen L (1992) Biochem Soc Trans 20:337–340
Yamada Y, Fujiwara T, Sato T, Igarashi N, Tanaka N (2002) Nat Struct Biol 9:691–695
Carpena X, Loprasert S, Mongkolsuk S, Switala J, Loewen PC, Fita I (2003) J Mol Biol 327:475–489
Wada K, Tada T, Nakamura Y, Kinoshita T, Tamoi M, Shigeoka S, Nishimura K (2002) Acta Cryst Section D58:157–159
Bertrand T, Eady NA, Jones JN, Jesmin, Nagy JM, Jamart-Gregoire B, Raven EL, Brown KA (2004) J Biol Chem 279:38991–38999
Jakopitsch C, Kolarich D, Petutschnig G, Furtmuller PG, Obinger C (2003) FEBS Lett 552:135–140
Marcinkeviciene JA, Magliozzo RS, Blanchard JS (1995) J Biol Chem 270:22290–22295
Johnsson K, Froland WA, Schultz PG (1997) J Biol Chem 272:2834–2840
Jakopitsch C, Ruker F, Regelsberger G, Dockal M, Peschek GA, Obinger C (1999) Biol Chem 380:1087–1096
Hillar A, Peters B, Pauls R, Loboda A, Zhang H, Mauk AJ, Loewen PC (2000) Biochemistry 39:5868–5875
Regelsberger G, Jakopitsch C, Ruker F, Krois D, Peschek GA, Obinger C (2000) J Biol Chem 275:22854–22861
Jakopitsch C, Auer M, Regelsberger G, Jantschko W, Furtmuller PG, Ruker F, Obinger C (2003) Biochemistry 42:5292–5300
Jakopitsch C, Auer M, Ivancich A, Ruker F, Furtmuller PG, Obinger C (2003) J Biol Chem 278:20185–20191
Hérouart D, Sigaud S, Moreau S, Frendo P, Touati D, Puppo A (1996) J Bacteriol 178:6802–6809
Sigaud S, Becquet V, Frendo P, Puppo A, Hérouart D (1999) J Bacteriol 181:2634–2639
Jamet A, Sigaud S, Van de Sype G, Puppo A, Hérouart D (2003) Mol Plant Microb Interact 16:217–225
Ardissone S, Frendo P, Laurenti E, Jantschko W, Obinger C, Puppo A, Ferrari RP (2004) Biochemistry 43:12692–12699
Yu S, Girotto S, Lee C, Magliozzo RS (2003) J Biol Chem 278:14769–14775
Casella L, Gullotti M, Poli S, Laurenti E, Ferrari RP, Marchesini A (1993) BioMetals 6:213–222
Beers RF, Sizer IV (1952) J Biol Chem 195:133–137
Nelson DP, Kiesow LA (1972) Anal Biochem 49:474–478
Ngo TT, Lenhoff HM (1980) Anal Biochem 105:389–397
Childs RE, Bardsley WG (1975) Biochem J 145:93–103
Ferrari RP, Ghibaudi EM, Traversa S, Laurenti E, De Gioia L, Salmona M (1997) J Inorg Biochem 68:17–26
Chouchane S, Girotto S, Kapetanaki S, Schelvis JPM, Yu S, Magliozzo RS (2003) J Biol Chem 278:8154–8162
Vitello LB, Huang M, Erman JE (1990) Biochemistry 29:4283–4288
Neri F, Kok D, Miller MA Smulevich G (1997) Biochemistry 36:8947–8953
Heering HA, Indiani C, Regelsberger G, Jakopitsch C, Obinger C, Smulevich G (2002) Biochemistry 41:9237–9247
Santoni E, Jakopitsch C, Obinger C, Smulevich G (2004) Biochemistry 43:5792–5802
Ghibaudi E, Laurenti E (2003) Eur J Biochem 270:4403–4412 and references therein
Pond AE, Sono M, Elenkova EA, McRee DE, Goodin DB, English AM, Dawson JH (1999) J Inorg Biochem 76:165–174
Smulevich G, Miller MA, Gosztola D, Spiro TG (1989) Biochemistry 28:9905–9908
Regelsberger G, Jakopitsch C, Engleder M, Ruker F, Peschek GA, Obinger C (1999) Biochemistry 38:10480–10488
Chouchane S, Girotto S, Yu S, Magliozzo RS (2002) J Biol Chem 277:42633–42638
Goodwin DC, Grover TA, Aust SD (1997) Biochemistry 36:139–147
Chouchane S, Lippai I, Magliozzo RS (2000) Biochemistry 39:9975–9983
Wengenack NL, Todorovic S, Yu L, Rusnak F (1998) Biochemistry 37:15825–15834
Jakopitsch C, Ivancich A, Schmuckenschlager F, Wanasinghe A, Poltl G, Furtmuller PG, Ruker F, Obinger C (2004) J Biol Chem 279:46082–46095
Roe JA, Goodin DB (1993) J Biol Chem 268:20037–20045
Kooter IM, Moguilevsky N, Bollen A, van der Veen LA, Otto C, Dekker HL, Wever R (1999) J Biol Chem 274:26794–26802
Kooter IM, Moguilevsky N, Bollen A, Sijtsema NM, Otto C, Wever R (1997) J Biol Inorg Chem 2:191–197
Kooter IM, Koehler BP, Moguilevsky N, Bollen A, Wever R, Johnson MK (1999) J Biol Inorg Chem 4:684–691
Sawyer DT (1988) In: Martell AE, Sawyer DT (eds) Oxygen complexes and oxygen activation by transition metals. Plenum Publishing Co, New York, pp 131
Monzani E, Gatti AL, Profumo A, Casella L, Gullotti M (1997) Biochemistry 36: 1918–1926
Yu S, Girotto S, Zhao X, Magliozzo RS (2003) J Biol Chem 278:44121–44127
Acknowledgements
The work was supported by a Ph.D. grant (to S.A.) from the ItalianMinistero per l’Istruzione, l’Università e la Ricerca. S.A. is grateful to R.P. Ferrari, who gave her access to the Bioinorganic Chemistry Laboratory of the University of Turin and allowed her to have all the necessary instrumental and technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ardissone, S., Laurenti, E., Frendo, P. et al. Single-site mutations on the catalase–peroxidase from Sinorhizobium meliloti: role of the distal Gly and the three amino acids of the putative intrinsic cofactor. J Biol Inorg Chem 10, 813–826 (2005). https://doi.org/10.1007/s00775-005-0032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0032-2