Abstract
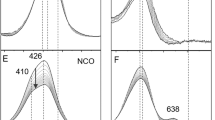
The coordination environment of the CuB center of the quinol oxidase from Acidianus ambivalens, a type B heme–copper oxygen reductase, was investigated by Fourier transform (FT) IR and extended X-ray absorption fine structure (EXAFS) spectroscopy. The comparative structural chemistry of dinuclear Fe–Cu sites of the different types of oxygen reductases is of great interest. Fully reduced A. ambivalens quinol oxidase binds CO at the heme a 3 center, with ν(CO)=1,973 cm−1. On photolysis, the CO migrated to the CuB center, forming a Cu IB –CO complex with ν(CO)=2,047 cm−1. Raising the temperature of the samples to 25°C did not result in a total loss of signal in the FTIR difference spectrum although the intensity of these signals was reduced sevenfold. This observation is consistent with a large energy barrier against the geminate rebinding of CO to the heme iron from CuB, a restricted limited access at the active-site pocket for a second binding, and a kinetically stable CuB–CO complex in A. ambivalens aa 3. The CuB center was probed in a number of different states using EXAFS spectroscopy. The oxidized state was best simulated by three histidines and a solvent O scatterer. On reduction, the site became three-coordinate, but in contrast to the bo 3 enzyme, there was no evidence for heterogeneity of binding of the coordinated histidines. The CuB centers in both the oxidized and the reduced enzymes also appeared to contain substoichiometric amounts (0.2 mol equiv) of nonlabile chloride ion. EXAFS data of the reduced carbonylated enzyme showed no difference between dark and photolyzed forms. The spectra could be well fit by 2.5 imidazoles, 0.5 Cl− and 0.5 CO ligands. This arrangement of scatterers would be consistent with about half the sites remaining as unligated Cu(his)3 and half being converted to Cu(his)2Cl−CO, a 50/50 ratio of Cu(his)2Cl− and Cu(his)3CO, or some combination of these formulations.








Similar content being viewed by others
References
Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikstrom M (2000) Nat Struct Biol 7:910–917
Iwata S, Ostermeier C, Ludwig B, Michel H (1995) Nature 376:660–669
Soulimane T, Buse G, Bourenkov GP, Bartunik HD, Huber R, Than ME (2000) EMBO J 19:1766–1776
Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S (1995) Science 269:1069–1074
Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S (2002) J Mol Biol 321:329–339
Pereira MM, Santana M, Teixeira M (2001) Biochim Biophys Acta 1505:185–208
Gomes CM, Backgren C, Teixeira M, Puustinen A, Verkhovskaya ML, Wikstrom M, Verkhovsky MI (2001) FEBS Lett 497:159–164
Bandeiras TM, Salgueiro C, Kletzin A, Gomes CM, Teixeira M (2002) FEBS Lett 531:273–277
Gomes CM, Lemos RS, Teixeira M, Kletzin A, Huber H, Stetter KO, Schafer G, Anemuller S (1999) Biochim Biophys Acta 1411:134–141
Lemos RS, Gomes CM, Teixeira M (2001) Biochem Biophys Res Commun 281:141–150
Anemüller S, Schmidt CL, Pacheco I, Schäfer G, Teixeira M (1994) FEMS Microbiol Lett 117:275–280
Purschke WG, Schmidt CL, Petersen A, Schafer G (1977) Bacteriol J 179:1344–1353
Giuffre A, Gomes CM, Antonini G, D’Itri E, Teixeira M, Brunori M (1997) Eur J Biochem 250:383–388
Fann YC, Ahmed I, Blackburn NJ, Boswell JS, Verkhovskaya ML, Hoffman BM, Wikstrom M (1995) Biochemistry 34:10245–10255
Teixeira M, Batista R, Campos AP, Gomes C, Mendes J, Pacheco I, Anemuller S, Hagen WR (1995) Eur J Biochem 227:322–327
Blackburn NJ, Rhames FC, Ralle M, Jaron S (2000) J Biol Inorg Chem 5:341–353
Eisses JF, Stasser JP, Ralle M, Kaplan J, Blackburn NJ (2000) Biochemistry 39:7337–7342
George GN (1995) EXAFSPAK, Stanford Synchrotron Radiation Laboratory
Binsted N, Gurman SJ, Campbell JW (1998) Daresbury Laboratory EXCURV98 Program
Das TK, Gomes CM, Bandeiras TM, Pereira MM, Teixeira M, Rousseau DL (2004) Biochim Biophys Acta 1655:306–320
Pereira MM, Teixeira M (2004) Biochim Biophys Acta 1655:340–346
Einarsdottir O, Killough PM, Fee JA, Woodruff WH (1989) J Biol Chem 264:2405–2408
Koutsoupakis K, Stavrakis S, Pinakoulaki E, Soulimane T, Varotsis C (2002) J Biol Chem 277:32860–32866
Aagaard A, Gilderson G, Gomes CM, Teixeira M, Brzezinski P (1990) Biochemistry 38:10032–10041
Pasquali M, Floriani C (1984) In: Karlin KD, Zubieta J (eds) Copper coordination chemistry, biochemical and inorganic perspectives. Adenine, New York, pp 311–330
Patch MG, Choi H, Chapman DR, Bau R, McKee V, Reed CA (1990) Inorg Chem 29:110–119
Villacorta GM, Lippard SJ (1987) Inorg Chem 26:3672–3676
Sorrell TN, Malachowski MR (1983) Inorg Chem 22:1883–1887
Sorrell TN, Borovick AS (1987) J Am Chem Soc 109:4255–4260
Ralle M, Verkovskaya ML, Morgan JE, Verkovsky MI, Wikstrom M, Blackburn NJ (1999) Biochemistry 38:7185–7194
Ostermeir C, Harrenga A, Ermler U, Michel H (1997) Proc Natl Acad Sci USA 94:10547–10533
Blackburn NJ, Barr ME, Woodruff WH, van der Oost J, de Vries S (1994) Biochemistry 33:10401–10407
Blackburn NJ, de Vries S, Barr ME, Houser RP, Tolman WB, Sanders D, Fee JA (1997) J Am Chem Soc 119:6135–6143
Blackburn NJ, Ralle M, Gomez E, Hill MG, Patsuszyn A, Sanders D, Fee JA (1999) Biochemistry 38:7075–7084
Osbourne JP, Cosper NJ, Stälhandske CMV, Scott RA, Alben JO, Gennis RB (1999) Biochemisty 38:4526–4532
Alben JO, Moh PP, Fiamingo FG, Altschuld RA (1981) Proc Natl Acad Sci USA 78:234–237
Dyer RB, Einarsdottir O, Killough PM, Lopez GJJ, Woodruff WH (1989) J Am Chem Soc 111:7657–7659
Puustinen A, Bailey JA, D. R.B., Mecklenburg SL, Wikstrom M, Woodruff WH (1997) Biochemistry 36:13195–13200
Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaona R, Yoshikawa S (1996) Science 272:1136–1144
Giuffre A, Forte E, Antonini G, D’Itri E, Brunori M, Soulimane T, Buse G (1999) Biochemistry 38:1057–1065
Moody AJ, Butler CS, Watmough NJ, Thomson AJ, Rich PR (1998) Biochem J 331:459–464
Butler CS, Seward HE, Greenwood C, Thomson AJ (1997) Biochemistry 36:16259–16266
Kau LS, Spira-Solomon D, Penner-Hahn JE, Hodgson KO, Solomon EI (1987) J Am Chem Soc 109:6433–6422
Pettingill TM, Strange RW, Blackburn NJ (1991) J Biol Chem 266:16996–17003
Blackburn NJ, Strange RW, Reedijk J, Volbeda A, Farooq A, Karlin KD, Zubieta J (1989) Inorg Chem 28:1349–1357
Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T (1998) Science 280:1723–1729
Harrenga A, Michel H (1999) J Biol Chem 274:33296–33299
Wikstrom M (2000) Biochim Biophys Acta 1458:188–198
Popovic DM, Stuchebrukhov AA (2004) FEBS Lett 566:126–130
Acknowledgements
The work was supported by a grant from the National Institutes of Health GM54803 to N.J.B. SSRL is supported by the National Institutes of Health Biomedical Research Technology Division, Division of Research Resources, and by the US Department of Energy, Basic Energy Sciences (BES), and Office of Biological and Environmental Research. The work was supported in part by a grant from FCT, project POCTI/BME/45122/2002. T.M.B. and M.M.P. are recipients of grants from the PRAXIS XXI program (BD/3133/00 and BPD/11621/2002). We acknowledge the contribution of C.M. Gomes (ITQB) at the early stages of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bandeiras, T.M., Pereira, M.M., Teixeira, M. et al. Structure and coordination of CuB in the Acidianus ambivalens aa 3 quinol oxidase heme–copper center. J Biol Inorg Chem 10, 625–635 (2005). https://doi.org/10.1007/s00775-005-0012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0012-6