Abstract
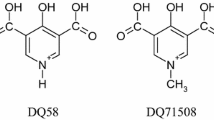
A series of extra-functionalized 3-hydroxy-4-pyridinone chelators of hard metal ions, containing different side-chains with peptidomimetic groups, was studied to assess the effect of those groups on the physico-chemical properties, the metal-chelating affinity and the in vivo behaviour of the compounds, in view of their potential pharmaceutical applications. Besides the synthesis of the chelators, the study of their properties in aqueous solution alone and in the presence of M 3+ (M = Fe, Ga and Al) was performed by potentiometric/spectroscopic techniques. The octanol/water partition coefficient values of these hydroxypyridinone derivatives cover ca. 3 orders of magnitude (1.1 > log P > −2). They all form very stable tris-chelated M(III) complexes, the pFe and pGa values ranging up to five orders of magnitude. The in vivo studies showed the effect of the ligands on the biodistribution of 67Ga citrate and also of 67Ga-complexes in mice, in view of the potential use of the ligands or complexes as metal decorporating or as imaging agents, respectively. Although almost all these peptidomimetic hydroxypyridinone derivatives present very rapid clearance rate from most organs, the L-ornithine derivative (H2L9) shows to be superior to the others and as good as Deferiprone as metal decontaminant of Ga. Concerning the 67Ga complexes, the benzyl-propylamine (H2L3) shows considerable bone retention, thus suggesting its potential application as imaging agent.








Similar content being viewed by others
References
Brock JH, Halliday JW, Pippard MJ (eds) (1994) Secondary iron overload. In: Iron metabolism in health and disease. W B Saunders Co Ltd, London, pp 271–309
Critchton RR, Florence A, Ward RJ (2002) Coord Chem Rev 228:365–371
Anderson CJ, Welch MJ (1999) Chem Rev 99:2219–2234
Faa G, Crisponi G (1999) Coord Chem Rev 184:291–310
Liu ZD, Hider RC (2002) Med Res Rev 22:26–64
Yokel RA (2002) Coord Chem Rev 228:97–113
Porter JB, Huehns ER (1989) Ballieres Clin Hematol 2:459–474
Reichert DE, Lewis JS, Anderson CJ (1999) Coord Chem Rev 184:3–66
Kontoghiorghes GJ (1985) Lancet 1:817
Hider RC, Liu ZD (2003) Curr Med Chem 10:1051–1064
Gomez M, Esparza JI, Domingo JL, Corbella J, Singh PK, Jones MM (1998) Pharmacol Toxicol 82:295–300
Shin RW, Kruck TPA, Murayama HM, Kitamoto T (2003) Brain Res 961:139–146
Yokel R, Datta AK, Jackson EG (1991) J Pharmacol Exp Therapeutics 257:100–106
Zhang Z, Lyster DM, Webb GA, Orvig C (1992) Nucl Med Chem 19:327–335
Ellis BL, Sampson CB, Abeysinghe rd, Porter JB, Hider RC (1999) Eur J Nucl Med 26:1400–1406
Santos MA (2002) Coord Chem Rev 228:187–203
Chaves S, Gil M, Marques S, Gano L, Santos MA (2003) J Inorg Biochem 97:161–172
Santos MA, Gama S, Gano L, Cantinho G, Farkas E (2004) J Chem Soc Dalton Trans 21:3772–3781
Santos MA, Gil M, Marques S, Gano L, Cantinho G, Chaves S (2002) J Inorg Biochem 92:43–54
Armarego WLF, Perring DD (eds) (1999) Purification of laboratory chemicals, 4th edn. Butterworth–Heinemann Press, Oxford
Santos MA, Grazina R, Neto AQ, Cantinho G, Gano L, Patrício L (2000) J Inorg Biochem 78:303–311
Rossotti FJC, Rossotti H (1965) J Chem Ed 42:375–378
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Öhman LO, Forschling W (1981) Acta Chem Scand Ser A 35:795–802
Baes CF, Mesmer RE (eds) (1976) The hydrolysis of cations. Wiley, New York
Covington AK, Paabo M, Robinson RA, Bates RG (1968) Anal Chem 40:700–706
Zékány L, Nagypál I, Peintler G (2001) PSEQUAD 501
Delgado R, Fraústo da Silva JJR, Amorim MTS, Cabral MF, Chaves S, Costa J (1991) Anal Chim Acta 245:271–282
Leo A, Hansch C, Elkins D (1971) Chem Rev 71:526–616
Rai BL, Dehhordi LS, Khodr H, Jin Y, Liu Z, Hider RC (1998) J Med Chem 41:3347–3358
John CS, Vilmer BJ, Geyer BC, Moody T, Bowen WD (1999) Cancer Res 59:4578–4583
de Costa BR, Radescu L, Di Paolo L, Bowen WD (1992) J Med Chem 35:38–47
Bedurftz S, Wunsch B (2004) Bioorg Med Chem 12:3299–3311
Menegatti R, Cunha AC, Ferreira VF, Perreira EFR, El-Nabawi A, Eldefrawi AT, Albuquerque EX, Neves G, Rates SMK, Fraga CAM, Barreiro EJ (2003) Bioorg Med Chem 11:4807–4813
Santos MA, Gil M, Marques S, Gano L, Chaves S (2005) Anal Bioanal Chem 381:413–419
Clarke ET, Martell AE (1992) Inorg Chim Acta 196:185–194
Martell AE, Motekaitis RJ (eds) (1988) Determination and use of stability constants. First supplement, VCH, New York, pp 428–429
Zhang Z, Rettig SJ, Orvig C (1991) Inorg Chem 30:509–515
Xiao G, Helm D, Hider, RC, Dobbin PS (1992) J Chem Soc Dalton Trans 3265–3271
Nelson WO, Rettig SJ, Orvig C (1989) Inorg Chem 28:3153–3157
Martell AE, Smith RM (eds) (1977) Critical stability constants, vol 3. Plenum Press, New York, pp 163
Gunasekera SW, King LJ, Lavender PJ (1972) Clin Chim Acta 39:401–406
Larson S, Rasey J, Allen D, Grunbaum DZ (1979) J Nucl Med 20:843–946
Hara T (1974) Int J Nucl Med Biol 1:152–154
Harris HR, Pecoraro VL (1983) Biochem 22:292
Acknowledgements
The authors thank the Portuguese Fundação para a Ciência e Tecnologia (FCT) (project POCTI/35344/99) and COST D21/001 program) for financial support. We also thank Dr Guilhermina Cantinho, Instituto de Medicina Nuclear, Faculdade de Medicina de Lisboa, for her support and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00775-006-0149-y
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Santos, M.A., Gil, M., Gano, L. et al. Bifunctional 3-hydroxy-4-pyridinone derivatives as potential pharmaceuticals: synthesis, complexation with Fe(III), Al(III) and Ga(III) and in vivo evaluation with 67Ga. J Biol Inorg Chem 10, 564–580 (2005). https://doi.org/10.1007/s00775-005-0003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0003-7