Abstract:
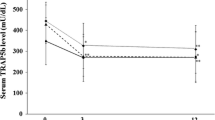
We investigated, in a multicenter study, the efficacy and safety of long-term administration of falecalcitriol, a new active vitamin D3, in patients with renal osteodystrophy of the osteitis fibrosa type associated with secondary hyperparathyroidism caused by chronic renal failure. Falecalcitriol was orally administered every day for 48 weeks. Administration was started at a dosage of 0.3 μg/day, and the dosage was changed whenever necessary according to serum calcium (Ca) level. As a result, significant inhibition of the bone resorption markers, i.e., intact parathyroid hormone (i-PTH), pyridinoline (Pyr), and deoxypyridinoline (D-Pyr), was observed from the 8th week, and the bone formation markers, i.e., total activity and bone fraction of alkaline phosphatase, were also significantly inhibited from the 12th week. The bone mineral density (BMD) change rate in the bones of the whole body determined by dual-energy X-ray absorptiometry remained almost constant. When subjects were stratified according to the inhibition rate of bone metabolic parameters, BMD tended to increase in the group with strong inhibition and to decrease in the group with weak inhibition. Mean serum Ca level significantly increased from 9.5 mg/dl, but mean level was subsequently maintained at about 10 mg/dl until the end of administration by adjustment of the doses. These findings suggested that falecalcitriol may inhibit and normalize accelerated bone metabolic turnover without inducing excessive increases in serum Ca level in secondary hyperparathyroidism. With respect to safety, no specific adverse reactions associated with the prolonged administration period were observed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: July 1, 1997 / Accepted: Aug. 29, 1997
About this article
Cite this article
Morii, H., Inoue, T., Fukunaga, M. et al. Efficacy and safety of long-term oral falecalcitriol treatment in patients with renal osteodystrophy. J Bone Miner Metab 16, 44–54 (1998). https://doi.org/10.1007/s007740050027
Issue Date:
DOI: https://doi.org/10.1007/s007740050027