Abstract
Introduction
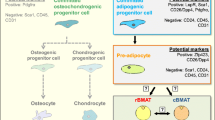
Low energy availability due to excessive exercise lowers bone mass and impairs various physiological functions, including immunity and hematopoiesis. We focused on Cxcl12 abundant reticular (CAR) cells, which are bone marrow mesenchymal stem cells and are essential for the maintenance of hematopoietic and immune cells in bone marrow. We examine the functional changes in CAR cells resulting from dietary restriction combined with exercise.
Materials and methods
Five-week-old wild-type female mice were divided into an ad libitum group (CON), a 60% dietary restriction group (DR), an ad libitum with exercise group (CON + ex), and a 60% dietary restriction with exercise group (DR + ex). Blood parameters, bone structure parameters, and bone marrow fat volume were evaluated after 5 weeks. In addition, bone marrow CAR cells were isolated by cell sorting and analyzed for gene expression by RT-qPCR.
Results
Bone mineral density (BMD) was significantly decreased in DR and DR + ex compared to CON and CON + ex. Especially, cortical bone mass and thickness were significantly decreased in DR and DR + ex groups, whereas trabecular bone mass was significantly increased. Bone marrow fat volume was significantly increased in DR and DR + ex groups compared to CON and CON + ex. The number of leukocytes in the blood was significantly decreased in the DR + ex group compared to the other three groups. RT-qPCR showed a significant decrease in gene expression of both Foxc1 and Runx2 in CAR cells of the DR + ex group compared to CON.
Conclusion
Dietary restriction combined with exercise promotes CAR cell differentiation into bone marrow adipocyte and suppresses osteoblast differentiation.





Similar content being viewed by others
References
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) American College of Sports Medicine position stand: physical activity and bone health. Med Sci Sports Exerc 36:1985–1996. https://doi.org/10.1249/01.mss.0000142662.21767.58
Hind K, Truscott JG, Evans JA (2006) Low lumbar spine bone mineral density in both male and female endurance runners. Bone 39:880–885. https://doi.org/10.1016/j.bone.2006.03.012
Bilanin JE, Blanchard MS, Russek-Cohen E (1989) Lower vertebral bone density in male long distance runners. Med Sci Sports Exerc 21:66–70. https://doi.org/10.1249/00005768-198902000-00012
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP (2007) American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 39:1867–1882. https://doi.org/10.1249/mss.0b013e318149f111
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A (2014) The IOC consensus statement: beyond the female athlete triad-relative energy deficiency in sport (RED-S). Br J Sports Med 48:491–497. https://doi.org/10.1136/bjsports-2014-093502
Ito E, Sato Y, Kobayashi T, Nakamura S, Kaneko Y, Soma T, Matsumoto T, Kimura A, Miyamoto K, Matsumoto H, Matsumoto M, Nakamura M, Sato K, Miyamoto T (2021) Food restriction reduces cortical bone mass and serum insulin-like growth factor-1 levels and promotes uterine atrophy in mice. Biochem Biophys Res Commun 534:165–171. https://doi.org/10.1016/j.bbrc.2020.11.122
Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB (2007) MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647. https://doi.org/10.1007/s00198-006-0285-9
Liu L, Rosen CJ (2023) New insights into calorie restriction induced bone loss. Endocrinol Metab 38:203–213. https://doi.org/10.3803/EnM.2023.1673
Li Z, Bowers E, Zhu J, Yu H, Hardij J, Bagchi DP, Mori H, Lewis KT, Granger K, Schill RL, Romanelli SM, Abrishami S, Hankenson KD, Singer K, Rosen CJ, MacDougald OA (2022) Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. Elife 11:e78496. https://doi.org/10.7554/eLife.78496
McGrath C, Sankaran JS, Misaghian-Xanthos N, Sen B, Xie Z, Styner MA, Zong X, Rubin J, Styner M (2020) Exercise degrades bone in caloric restriction, despite suppression of marrow adipose tissue (MAT). J Bone Miner Res 35:106–115. https://doi.org/10.1002/jbmr.3872
Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA (2018) Development, regulation, metabolism and function of bone marrow adipose tissues. Bone 110:134–140. https://doi.org/10.1016/j.bone.2018.01.008
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, Saraiva LR, Schulz TJ (2017) Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20:771-784.e776. https://doi.org/10.1016/j.stem.2017.02.009
Fazeli PK, Bredella MA, Pachon-Pena G, Zhao W, Zhang X, Faje AT, Resulaj M, Polineni SP, Holmes TM, Lee H, O’Donnell EK, MacDougald OA, Horowitz MC, Rosen CJ, Klibanski A (2021) The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight 6:e138636. https://doi.org/10.1172/jci.insight.138636
Omatsu Y, Nagasawa T (2021) Identification of microenvironmental niches for hematopoietic stem cells and lymphoid progenitors-bone marrow fibroblastic reticular cells with salient features. Int Immunol. https://doi.org/10.1093/intimm/dxab092
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168. https://doi.org/10.1016/j.stem.2014.06.008
Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T (2010) The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33:387–399. https://doi.org/10.1016/j.immuni.2010.08.017
Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T (2018) Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev 32:359–372. https://doi.org/10.1101/gad.311068.117
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, Nelson T, Katz G, Berry R, Church CD, Doucette CR, Rodeheffer MS, Macdougald OA, Rosen CJ, Horowitz MC (2014) Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol 537:123–139. https://doi.org/10.1016/B978-0-12-411619-1.00007-0
Polineni S, Resulaj M, Faje AT, Meenaghan E, Bredella MA, Bouxsein M, Lee H, MacDougald OA, Klibanski A, Fazeli PK (2020) Red and white blood cell counts are associated with bone marrow adipose tissue, bone mineral density, and bone microarchitecture in premenopausal women. J Bone Miner Res 35:1031–1039. https://doi.org/10.1002/jbmr.3986
Goldberg EL, Dixit VD (2019) Bone Marrow: An Immunometabolic Refuge during Energy Depletion. Cell Metab 30:621–623. https://doi.org/10.1016/j.cmet.2019.08.022
Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, Kishton RJ, Shannon JP, Dixit D, Schwab SR, Hickman HD, Restifo NP, McGavern DB, Schwartzberg PL, Belkaid Y (2019) The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178:1088-1101.e1015. https://doi.org/10.1016/j.cell.2019.07.049
Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, Khoury B, Jepsen KJ, Pilch PF, Klibanski A, Rosen CJ, MacDougald OA (2015) Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:7808. https://doi.org/10.1038/ncomms8808
Devlin MJ, Rosen CJ (2015) The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol 3:141–147. https://doi.org/10.1016/s2213-8587(14)70007-5
Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T (2014) Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 508:536–540. https://doi.org/10.1038/nature13071
Aaron N, Kraakman MJ, Zhou Q, Liu Q, Costa S et al (2021) Adipsin promotes bone marrow adiposity by priming mesenchymal stem cells. Elife 10:e69209. https://doi.org/10.7554/eLife.69209
Wan Y, Chong LW, Evans RM (2007) PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 13:1496–1503. https://doi.org/10.1038/nm1672
Tzeng YS, Chung NC, Chen YR, Huang HY, Chuang WP, Lai DM (2018) Imbalanced osteogenesis and adipogenesis in mice deficient in the chemokine Cxcl12/Sdf1 in the bone mesenchymal stem/progenitor cells. J Bone Miner Res 33:679–690. https://doi.org/10.1002/jbmr.3340
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764. https://doi.org/10.1016/s0092-8674(00)80258-5
Omatsu Y, Aiba S, Maeta T, Higaki K, Aoki K, Watanabe H, Kondoh G, Nishimura R, Takeda S, Chung UI, Nagasawa T (2022) Runx1 and Runx2 inhibit fibrotic conversion of cellular niches for hematopoietic stem cells. Nat Commun 13:2654. https://doi.org/10.1038/s41467-022-30266-y
Acknowledgements
The authors thank the staff of the Division of Analytical Bio-Medicine and the Division of Laboratory Animal Research, the Advanced Research Support Center (ADRES), the members of the Division of Integrative Pathophysiology, Proteo-Science Center (PROS), and Ehime University for their technical assistance and helpful support.
Funding
This work was supported by the 37th Research Grant of Meiji Yasuda Life Foundation of Health and Welfare.
Author information
Authors and Affiliations
Contributions
Investigation: AI; conceptualization: AI, YI; data curation: AI; funding acquisition: AI; supervision: YI; writing: AI, YI.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ikedo, A., Imai, Y. Dietary restriction plus exercise change gene expression of Cxcl12 abundant reticular cells in female mice. J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01506-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01506-6