Abstract
Introduction
The increase of ATP concentration in the extracellular space represents one of the effective signals that stimulate the physiological activities of cells when the bone is exposed to external mechanical stimulation such as stretching and shear stress force throughout life. However, the effects of ATP on osteoblast differentiation and related mechanisms are not well understood.
Materials and Methods
In this study, the roles of extracellular ATP on osteoblast differentiation, intracellular calcium ([Ca2+]i) levels, metabolomics, and the expression of proteins related to energy metabolism were investigated.
Results
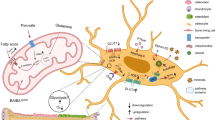
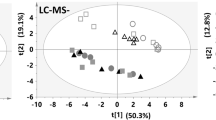
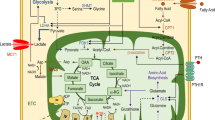
Our results showed that 100 μM extracellular ATP initiated intracellular calcium ([Ca2+]i) oscillations via the calcium-sensing receptor (P2R) and promoted the differentiation of MC3T3-E1 cells. Metabolomics analysis showed that the differentiation of MC3T3-E1 cells depended on aerobic oxidation, but little glycolysis. Moreover, the differentiation of MC3T3-E1 cells and aerobic oxidation were suppressed with the inhibition of AMP-activated protein kinase (AMPK).
Conclusion
These results indicate that calcium oscillations triggered by extracellular ATP can activate aerobic oxidation through AMPK-related signaling pathways and thus promote osteoblast differentiation.







Similar content being viewed by others
References
Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol 220:155–162. https://doi.org/10.1002/jcp.21745
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ (2007) Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212:207–214. https://doi.org/10.1002/jcp.21021
Mikolajewicz N, Zimmermann EA, Willie BM, Komarova SV (2018) Mechanically stimulated ATP release from murine bone cells is regulated by a balance of injury a repair (in English). Elife 7:e37812. https://doi.org/10.7554/eLife.37812
Verkhratsky A, Burnstock G (2014) Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 36:697–705. https://doi.org/10.1002/bies.201400024
Zhang WY, Dong ZW, Li DK, Li B, Liu Y, Zheng XN, Liu H, Zhou HZ, Hu KJ, Xue Y (2021) Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway (in English). Cell Proliferat 54:e13058. https://doi.org/10.1111/cpr.13058
Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T (2016) Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife. https://doi.org/10.7554/eLife.13374
Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M et al (2015) Metabolic programming and PDHK1 control CD4(+) T cell subsets and inflammation (in English). J Clin Invest 125:194–207. https://doi.org/10.1172/Jci76012
Li B, Lee WC, Song C, Ye L, Abel ED, Long F (2020) Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. Faseb J 34:11058–11067. https://doi.org/10.1096/fj.202000771R
Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, Xu J, Chen L, Cao K, Long J, Li Z, Shen W, Liu J (2018) SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ 25:229–240. https://doi.org/10.1038/cdd.2017.144
Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG, Brand MD (2015) The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 1847:171–181. https://doi.org/10.1016/j.bbabio.2014.10.005
Malgaroli A, Milani D, Meldolesi J, Pozzan T (1987) Fura-2 measurement of cytosolic free Ca-2+ in monolayers and suspensions of various types of animal-cells (in English). J Cell Biol 105:2145–2155. https://doi.org/10.1083/jcb.105.5.2145
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Yoshida H, Kobayashi D, Ohkubo S, Nakahata N (2006) ATP stimulates interleukin-6 production via P2Y receptors in human HaCaT keratinocytes. Eur J Pharmacol 540:1–9. https://doi.org/10.1016/j.ejphar.2006.04.008
Tsuchiya N, Kodama D, Goto S, Togari A (2015) Shear stress-induced Ca(2+) elevation is mediated by autocrine-acting glutamate in osteoblastic MC3T3-E1 cells. J Pharmacol Sci 127:311–318. https://doi.org/10.1016/j.jphs.2015.01.005
Li ZQ, Liu TJ, Gilmore A, Gomez NM, Mitchell CH, Li YP, Oursler MJ, Yang SY (2018) Regulator of G protein signaling protein 12 is required for osteoblast differentiation through controlling calcium channel/G alpha i-calcium oscillation-ERK signaling (in English). J Bone Miner Res 33:93–93
Keinan D, Yang SY, Cohen RE, Yuan X, Liu TJ, Li YP (2014) Role of regulator of G protein signaling proteins in bone (in English). Front Biosci-Landmark 19:634–648. https://doi.org/10.2741/4232
Vultaggio-Poma V, Sarti AC, Di Virgilio F (2020) Extracellular ATP: a feasible target for cancer therapy (in English). Cells-Basel 9:2496
Hecht E, Liedert A, Ignatius A, Mizaikoff B, Kranz C (2013) Local detection of mechanically induced ATP release from bone cells with ATP microbiosensors. Biosens Bioelectron 44:27–33. https://doi.org/10.1016/j.bios.2013.01.008
Rumney RM, Sunters A, Reilly GC, Gartland A (2012) Application of multiple forms of mechanical loading to human osteoblasts reveals increased ATP release in response to fluid flow in 3D cultures and differential regulation of immediate early genes. J Biomech 45:549–554. https://doi.org/10.1016/j.jbiomech.2011.11.036
Noronha-Matos JB, Correia-de-Sa P (2016) Mesenchymal stem cells ageing: targeting the “Purinome” to promote osteogenic differentiation and bone repair (in English). J Cell Physiol 231:1852–1861. https://doi.org/10.1002/jcp.25303
Graneli C, Thorfve A, Ruetschi U, Brisby H, Thomsen P, Lindahl A, Karlsson C (2014) Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res 12:153–165. https://doi.org/10.1016/j.scr.2013.09.009
Li Q, Gao Z, Chen Y, Guan MX (2017) The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 8:439–445. https://doi.org/10.1007/s13238-017-0385-7
Shen YL, Wu L, Wang J, Wu X, Zhang XM (2018) The role of mitochondria in methamphetamine-induced inhibitory effects on osteogenesis of mesenchymal stem cells (in English). Eur J Pharmacol 826:56–65. https://doi.org/10.1016/j.ejphar.2018.02.049
Guntur AR, Le PT, Farber CR, Rosen CJ (2014) Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology 155:1589–1595. https://doi.org/10.1210/en.2013-1974
Lee WC, Guntur AR, Long F, Rosen CJ (2017) Energy metabolism of the osteoblast: implications for osteoporosis. Endocr Rev 38:255–266. https://doi.org/10.1210/er.2017-00064
Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang YF, Linehan WM, Chandel NS, DeBerardinis RJ (2012) Reductive carboxylation supports growth in tumour cells with defective mitochondria (in English). Nature 481:385-U171. https://doi.org/10.1038/nature10642
Belevich I, Verkhovsky MI, Wikstrom M (2006) Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase (in English). Nature 440:829–832. https://doi.org/10.1038/nature04619
Garcia-Heredia JM, Carnero A (2015) Decoding Warburg’s hypothesis: tumor-related mutations in the mitochondrial respiratory chain (in English). Oncotarget 6:41582–41599. https://doi.org/10.18632/oncotarget.6057
Henriksen Z, Hiken JF, Steinberg TH, Jorgensen NR (2006) The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells (in English). Cell Calcium 39:435–444. https://doi.org/10.1016/j.ceca.2006.01.012
Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR (2006) Osteoblast responses to nucleotides increase during differentiation. Bone 39:300–309. https://doi.org/10.1016/j.bone.2006.02.063
Strohbach CA, Genetos DC, Taylor AF, Donahue HJ (2004) Differentiation affects MC3T3-E1 mechanoresponsiveness and P2Y2 expression. J Bone Miner Res 19:S257
Smedler E, Uhlen P (2014) Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840:964–969. https://doi.org/10.1016/j.bbagen.2013.11.015
Salazar C, Politi AZ, Hofer T (2008) Decoding of calcium oscillations by phosphorylation cycles: analytic results. Biophys J 94:1203–1215. https://doi.org/10.1529/biophysj.107.113084
Dupont G, Houart G, De Koninck P (2003) Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium 34:485–497. https://doi.org/10.1016/s0143-4160(03)00152-0
Munaron L, Antoniotti S, Lovisolo D (2004) Intracellular calcium signals and control of cell proliferation: how many mechanisms? (in English). J Cell Mol Med 8:161–168. https://doi.org/10.1111/j.1582-4934.2004.tb00271.x
Scharenberg AM, Humphries LA, Rawlings DJ (2007) Calcium signalling and cell-fate choice in B cells (in English). Nat Rev Immunol 7:778–789. https://doi.org/10.1038/nri2172
Sun S, Liu YM, Lipsky S, Cho M (2007) Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells (in English). Faseb J 21:1472–1480. https://doi.org/10.1096/fj.06-7153com
Tseng KY, Wang HC, Chang LL, Cheng KI (2018) Advances in experimental medicine and biology: intrafascicular local anesthetic injection damages peripheral nerve-induced neuropathic pain. Adv Exp Med Biol 1099:65–76. https://doi.org/10.1007/978-981-13-1756-9_6
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling (in English). Nat Rev Mol Cell Bio 4:517–529. https://doi.org/10.1038/nrm1155
Dolmetsch RE, Xu KL, Lewis RS (1998) Calcium oscillations increase the efficiency and specificity of gene expression (in English). Nature 392:933–936. https://doi.org/10.1038/31960
Lieben L, Carmeliet G (2012) The involvement of TRP channels in bone homeostasis. Front Endocrinol (Lausanne) 3:99. https://doi.org/10.3389/fendo.2012.00099
Fioretti B, Pietrangelo T, Catacuzzeno L, Franciolini F (2005) Intermediate-conductance Ca2+-activated K+ channel is expressed in C2C12 myoblasts and is downregulated during myogenesis (in English). Am J Physiol-Cell Ph 289:C89–C96. https://doi.org/10.1152/ajpcell.00369.2004
Sato M, Asano T, Hosomichi J, Ono T, Nakata T (2018) Optogenetic manipulation of intracellular calcium by BACCS promotes differentiation of MC3T3-E1 cells. Biochem Biophys Res Commun 506:716–722. https://doi.org/10.1016/j.bbrc.2018.10.107
Toth AB, Shum AK, Prakriya M (2016) Regulation of neurogenesis by calcium signaling (in English). Cell Calcium 59:124–134. https://doi.org/10.1016/j.ceca.2016.02.011
Smedler E, Uhlen P (2014) Frequency decoding of calcium oscillations (in English). Bba-Gen Subjects 1840:964–969. https://doi.org/10.1016/j.bbagen.2013.11.015
Choi Y, Park JE, Jeong JS, Park JK, Kim J, Jeon S (2016) Sound waves induce neural differentiation of human bone marrow-derived mesenchymal stem cells via ryanodine receptor-induced calcium release and Pyk2 activation. Appl Biochem Biotechnol 180:682–694. https://doi.org/10.1007/s12010-016-2124-6
Hardie DG (2014) AMPK-sensing energy while talking to other signaling pathways (in English). Cell Metab 20:939–952. https://doi.org/10.1016/j.cmet.2014.09.013
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase (in English). Cell Metab 2:9–19. https://doi.org/10.1016/j.cmet.2005.05.009
Yi XS, Wang W, Xie QF (2018) Adenosine receptors enhance the ATP-induced odontoblastic differentiation of human dental pulp cells (in English). Biochem Bioph Res Co 497:850–856. https://doi.org/10.1016/j.bbrc.2018.02.125
Cutarelli A, Marini M, Tancredi V, D’Arcangelo G, Murdocca M, Frank C, Tarantino U (2016) Adenosine Triphosphate stimulates differentiation and mineralization in human osteoblast-like Saos-2 cells (in English). Dev Growth Differ 58:400–408. https://doi.org/10.1111/dgd.12288
Bell CJ, Bright NA, Rutter GA, Griffiths EJ (2006) Atp regulation in adult rat cardiomyocytes time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins (in English). J Biol Chem 281:28058–28067. https://doi.org/10.1074/jbc.M604540200
Arciuch VGA, Russo MA, Kang KS, Di Cristofano A (2013) Inhibition of AMPK and krebs cycle gene expression drives metabolic remodeling of pten-deficient preneoplastic thyroid cells (in English). Cancer Res 73:5459–5472. https://doi.org/10.1158/0008-5472.Can-13-1429
Izumiya M, Haniu M, Ueda K, Ishida H, Ma C, Ideta H, Sobajima A, Ueshiba K, Uemura T, Saito N, Haniu H (2021) Evaluation of MC3T3-E1 cell osteogenesis in different cell culture media. Int J Mol Sci. https://doi.org/10.3390/ijms22147752
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7:683–692. https://doi.org/10.1002/jbmr.5650070613
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11872297 and No. 31670954).
Author information
Authors and Affiliations
Contributions
JZ conceived and designed the research; XG, YK, and JL performed the experiments; XG, XD, and JL contributed to sample preparation and data analysis; LS, XD, and JL contributed to the interpretation of the results; XG wrote the original draft; WX and LS helped supervise the project and reviewed draft; JZ reviewed draft, acquired funding, and administrated the project. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest is declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Gao, X., Di, X., Li, J. et al. Extracellular ATP-induced calcium oscillations regulating the differentiation of osteoblasts through aerobic oxidation metabolism pathways. J Bone Miner Metab 41, 606–620 (2023). https://doi.org/10.1007/s00774-023-01449-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01449-4