Abstract
Introduction
As the population ages, the incidence of osteoporosis among patients suffering from Parkinson's disease (PD) will surge continually, and the ensuing disability from falls is becoming a serious social burden. Due to its antioxidant properties, much literature has indicated the possible ability of serum uric acid (UA) to prevent ageing-related diseases caused by oxidative stress, including osteoporosis and PD. Therefore, this study was for exploring the connection of serum UA levels with bone mineral density (BMD) and the osteoporosis presence in Chinese PD patients.
Materials and methods
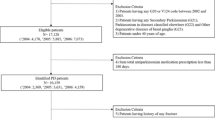
A cross-sectional design was used to statistically analyze 42 clinical parameters obtained from 135 patients with PD treated in Wuhan Tongji Hospital during 2020–2022. Multiple stepwise linear regression and multiple logistic regression analyses were constructed for identifying the association of serum UA levels with BMD as well as osteoporosis in PD patients, respectively. With receiver operative characteristic (ROC) curves, the optimal cutoff value was acquired for serum UA in the diagnosis of osteoporosis.
Results
According to the regression analysis adjusted for confounders, serum UA levels in PD patients had positive correlation with BMD at each site and negative correlation with the presence of osteoporosis (P < 0.05 for all). ROC curves determined that the optimal cutoff value for UA to perform well in diagnosing osteoporosis in PD patients was 284.27 μmol/L (P < 0.001).
Conclusion
Relatively higher serum UA levels in the physiological range can work as a biomarker of higher BMD, and were strongly linked to lower prevalence of osteoporosis in Chinese PD patients.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- PD:
-
Parkinson’s disease
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- ROS:
-
Reactive oxygen species
- UA:
-
Uric acid
- CKD:
-
Chronic kidney disease
- CKD–EPI:
-
CKD–Epidemiology
- T2DM:
-
Type 2 diabetes mellitus
- NHANES:
-
National health and nutrition examination survey
- UK-PDSBB:
-
United Kingdom Parkinson’s disease society brain bank
- Ca:
-
Calcium
- UPDRS:
-
Unified Parkinson's Disease Rating Scale
- H&Y stage:
-
Hoehn and Yahr stage
- ALP:
-
Alkaline phosphatase
- P:
-
Phosphorus
- BTMs:
-
Bone turnover markers
- TH:
-
Total hip
- FN:
-
Femoral neck
- LS:
-
Lumbar spine
- ALB:
-
Albumin
- GLB:
-
Globulin
- DBIL:
-
Direct bilirubin
- IBIL:
-
Indirect bilirubin
- Cr:
-
Creatinine
- LDH:
-
Lactate dehydrogenase
- BUN:
-
Blood urea nitrogen
- eGFR:
-
Estimated glomerular filtration rate
- NLR:
-
Neutrophil count/lymphocyte count
- MLR:
-
Monocyte count/lymphocyte count
- PLR:
-
Platelet count/lymphocyte count
- AGR:
-
ALB/GLB
- PINP:
-
Procollagen type I N-terminal pro-peptide
- OC:
-
Osteocalcin
- PTH:
-
Parathyroid hormone
- β-CTX:
-
β-C-terminal telopeptide of type I collagen
- 25(OH)VitD:
-
25-Hydroxyvitamin D
- DXA:
-
Dual-energy X-ray absorptiometry
- SD:
-
Standard deviation
- WHO:
-
World health organization
- OR:
-
Odds ratio
- CI:
-
Confidence intervals
- ROC:
-
Receiver operative characteristic
- AUC:
-
Area under curve
References
Lim SY, Tan AH, Ahmad-Annuar A, Klein C, Tan LCS et al (2019) Parkinson’s disease in the Western Pacific Region. Lancet Neurol 18:865–879. https://doi.org/10.1016/S1474-4422(19)30195-4
Abou-Raya S, Helmii M, Abou-Raya A (2009) Bone and mineral metabolism in older adults with Parkinson’s disease. Age Ageing 38:675–680. https://doi.org/10.1093/ageing/afp137
Lee A, Gilbert RM (2016) Epidemiology of Parkinson disease. Neurol Clin 34:955–965. https://doi.org/10.1016/j.ncl.2016.06.012
Tan AH, Hew YC, Lim SY, Ramli NM, Kamaruzzaman SB et al (2018) Altered body composition, sarcopenia, frailty, and their clinico-biological correlates, in Parkinson’s disease. Parkinsonism Relat Disord 56:58–64. https://doi.org/10.1016/j.parkreldis.2018.06.020
van den Bos F, Speelman AD, Samson M, Munneke M, Bloem BR, Verhaar HJ (2013) Parkinson’s disease and osteoporosis. Age Ageing 42:156–162. https://doi.org/10.1093/ageing/afs161
Torsney KM, Noyce AJ, Doherty KM, Bestwick JP, Dobson R, Lees AJ (2014) Bone health in Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 85:1159–1166. https://doi.org/10.1136/jnnp-2013-307307
Ensrud KE, Crandall CJ (2017) Osteoporosis. Ann Internal Med 167:ITC17–ITC32. https://doi.org/10.7326/AITC201708010
Choi SM, Cho SH, Kim BC (2021) Association between freezing of gait and bone mineral density in patients with Parkinson’s disease. Neurol Sci 42:2921–2925. https://doi.org/10.1007/s10072-020-04920-6
Muhlenfeld N, Sohling N, Marzi I, Pieper M, Paule E et al (2021) Fractures in Parkinson’s Disease: injury patterns, hospitalization, and therapeutic aspects. Eur J Trauma Emerg Surg 47:573–580. https://doi.org/10.1007/s00068-019-01240-z
Sleeman I, Che ZC, Counsell C (2016) Risk of fracture amongst patients with Parkinson’s disease and other forms of parkinsonism. Parkinsonism Relat Disord 29:60–65. https://doi.org/10.1016/j.parkreldis.2016.05.026
Shribman S, Torsney KM, Noyce AJ, Giovannoni G, Fearnley J, Dobson R (2014) A service development study of the assessment and management of fracture risk in Parkinson’s disease. J Neurol 261:1153–1159. https://doi.org/10.1007/s00415-014-7333-8
Kimball JS, Johnson JP, Carlson DA (2021) Oxidative stress and osteoporosis. J Bone Joint Surg Am 103:1451–1461. https://doi.org/10.2106/JBJS.20.00989
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32. https://doi.org/10.1016/j.pneurobio.2013.04.004
Wei W, Ji S (2018) Cellular senescence: molecular mechanisms and pathogenicity. J Cell Physiol 233:9121–9135. https://doi.org/10.1002/jcp.26956
El Assar M, Angulo J, Rodriguez-Manas L (2013) Oxidative stress and vascular inflammation in aging. Free Radical Biol Med 65:380–401. https://doi.org/10.1016/j.freeradbiomed.2013.07.003
El Ridi R, Tallima H (2017) Physiological functions and pathogenic potential of uric acid: a review. J Adv Res 8:487–493. https://doi.org/10.1016/j.jare.2017.03.003
Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151. https://doi.org/10.2174/138161205774913255
Veronese N, Carraro S, Bano G, Trevisan C, Solmi M et al (2016) Hyperuricemia protects against low bone mineral density, osteoporosis and fractures: a systematic review and meta-analysis. Eur J Clin Invest 46:920–930. https://doi.org/10.1111/eci.12677
Sampat R, Young S, Rosen A, Bernhard D, Millington D et al (2016) Potential mechanisms for low uric acid in Parkinson disease. J Neural Transm (Vienna) 123:365–370. https://doi.org/10.1007/s00702-015-1503-4
Yan DD, Wang J, Hou XH, Bao YQ, Zhang ZL et al (2018) Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol Sin 39:626–632. https://doi.org/10.1038/aps.2017.165
Iki M, Yura A, Fujita Y, Kouda K, Tachiki T et al (2020) Relationships between serum uric acid concentrations, uric acid lowering medications, and vertebral fracture in community-dwelling elderly Japanese men: Fujiwara-kyo osteoporosis risk in men (FORMEN) cohort study. Bone 139:115519. https://doi.org/10.1016/j.bone.2020.115519
Ahn SH, Lee SH, Kim BJ, Lim KH, Bae SJ et al (2013) Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int 24:2961–2970. https://doi.org/10.1007/s00198-013-2377-7
Kaushal N, Vohora D, Jalali RK, Jha S (2018) Raised serum uric acid is associated with higher bone mineral density in a cross-sectional study of a healthy Indian population. Ther Clin Risk Manag 14:75–82. https://doi.org/10.2147/TCRM.S147696
Ibrahim WN, Younes N, Shi Z, Abu-Madi MA (2021) Serum uric acid level is positively associated with higher bone mineral density at multiple skeletal sites among healthy qataris. Frontiers Endocrinol 12:653685. https://doi.org/10.3389/fendo.2021.653685
Karimi F, Dabbaghmanesh MH, Omrani GR (2019) Association between serum uric acid and bone health in adolescents. Osteoporos Int 30:2057–2064. https://doi.org/10.1007/s00198-019-05072-w
Li X, Li L, Yang L, Yang J, Lu H (2021) No association between serum uric acid and lumbar spine bone mineral density in US adult males: a cross sectional study. Sci Rep 11:15588. https://doi.org/10.1038/s41598-021-95207-z
Zhang D, Bobulescu IA, Maalouf NM, Adams-Huet B, Poindexter J et al (2015) Relationship between serum uric acid and bone mineral density in the general population and in rats with experimental hyperuricemia. J Bone Miner Res 30:992–999. https://doi.org/10.1002/jbmr.2430
Afsar B, Sag AA, Oztosun C, Kuwabara M, Cozzolino M et al (2019) The role of uric acid in mineral bone disorders in chronic kidney disease. J Nephrol 32:709–717. https://doi.org/10.1007/s40620-019-00615-0
Yan P, Zhang Z, Wan Q, Zhu J, Li H et al (2018) Association of serum uric acid with bone mineral density and clinical fractures in Chinese type 2 diabetes mellitus patients: a cross-sectional study. Clinica Chimica Acta Int J Clin Chem 486:76–85. https://doi.org/10.1016/j.cca.2018.07.033
Wang J, Xie P, Huang JM, Qu Y, Zhang F et al (2016) The new Asian modified CKD-EPI equation leads to more accurate GFR estimation in Chinese patients with CKD. Int Urol Nephrol 48:2077–2081. https://doi.org/10.1007/s11255-016-1386-9
Delanaye P, Masson I, Maillard N, Pottel H, Mariat C (2022) The new 2021 CKD-EPI equation without race in a european cohort of renal transplanted patients. Transplantation 106:2443–2447. https://doi.org/10.1097/TP.0000000000004234
Zhao X, Yu X, Zhang X (2020) Association between uric acid and bone mineral density in postmenopausal women with type 2 diabetes mellitus in china: a cross-sectional inpatient study. J Diabetes Res 2020:3982831. https://doi.org/10.1155/2020/3982831
Zhao DD, Jiao PL, Yu JJ, Wang XJ, Zhao L et al (2016) Higher serum uric acid is associated with higher bone mineral density in chinese men with type 2 diabetes mellitus. Int J Endocrinol 2016:2528956. https://doi.org/10.1155/2016/2528956
Xu M, Su J, Hao J, Zhong N, Zhang Z et al (2018) Positive association between serum uric acid and bone mineral density in Chinese type 2 diabetes mellitus stratified by gender and BMI. J Bone Miner Metab 36:609–619. https://doi.org/10.1007/s00774-017-0877-9
Lin ZC, Wu JF, Chang CY, Lai KM, Yang HY (2022) Association between serum uric acid level and bone mineral density at multiple skeletal sites in middle-aged and elderly men: a cross-sectional study of a healthy population in Taiwan. Arch Osteoporos 17:142. https://doi.org/10.1007/s11657-022-01186-7
Xiao J, Chen W, Feng X, Liu W, Zhang Z et al (2017) Serum uric acid is associated with lumbar spine bone mineral density in healthy Chinese males older than 50 years. Clin Interv Aging 12:445–452. https://doi.org/10.2147/CIA.S130690
Chen F, Wang Y, Guo Y, Wang J, Yang A et al (2019) Specific higher levels of serum uric acid might have a protective effect on bone mineral density within a Chinese population over 60 years old: a cross-sectional study from northeast China. Clin Interv Aging 14:1065–1073. https://doi.org/10.2147/CIA.S186500
Pan K, Yao X, Liu M, Zhu Z (2020) Association of serum uric acid status with bone mineral density in adolescents aged 12–19 years. Front Med (Lausanne) 7:255. https://doi.org/10.3389/fmed.2020.00255
Hohn A, Tramutola A, Cascella R (2020) Proteostasis failure in neurodegenerative diseases: focus on oxidative stress. Oxid Med Cell Longev 2020:5497046. https://doi.org/10.1155/2020/5497046
Seifar F, Dinasarapu AR, Jinnah HA (2022) Uric acid in Parkinson’s disease: what is the connection? Mov Disord 37:2173–2183. https://doi.org/10.1002/mds.29209
Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jimenez-Urbieta H, Rodriguez-Oroz MC (2016) Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov Disord 31:861–881. https://doi.org/10.1002/mds.26662
van Wamelen DJ, Taddei RN, Calvano A, Titova N, Leta V et al (2020) Serum uric acid levels and non-motor symptoms in Parkinson’s disease. J Parkinsons Dis 10:1003–1010. https://doi.org/10.3233/JPD-201988
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359–370. https://doi.org/10.1007/s00774-015-0656-4
Rao AV, Balachandran B (2002) Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci 5:291–309. https://doi.org/10.1080/1028415021000033767
Li HZ, Chen Z, Hou CL, Tang YX, Wang F, Fu QG (2015) Uric acid promotes osteogenic differentiation and inhibits adipogenic differentiation of human bone mesenchymal stem cells. J Biochem Mol Toxicol 29:382–387. https://doi.org/10.1002/jbt.21707
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Investig 85:632–639. https://doi.org/10.1172/JCI114485
Steinbeck MJ, Appel WH Jr, Verhoeven AJ, Karnovsky MJ (1994) NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol 126:765–772. https://doi.org/10.1083/jcb.126.3.765
Lin KM, Lu CL, Hung KC, Wu PC, Pan CF et al (2019) The paradoxical role of uric acid in osteoporosis. Nutrients. https://doi.org/10.3390/nu11092111
Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G (2008) The paradoxical relationship between serum uric acid and cardiovascular disease. Clinica Chimica Acta Int J Clin Chem 392:1–7. https://doi.org/10.1016/j.cca.2008.02.024
Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G (1999) When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J 340:143–152
Sautin YY, Johnson RJ (2008) Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27:608–619. https://doi.org/10.1080/15257770802138558
Ishii S, Miyao M, Mizuno Y, Tanaka-Ishikawa M, Akishita M, Ouchi Y (2014) Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos Int 25:1099–1105. https://doi.org/10.1007/s00198-013-2571-7
Makovey J, Macara M, Chen JS, Hayward CS, March L et al (2013) Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: a longitudinal study. Bone 52:400–406. https://doi.org/10.1016/j.bone.2012.10.025
Pirro M, Mannarino MR, Bianconi V, De Vuono S, Sahebkar A et al (2017) Uric acid and bone mineral density in postmenopausal osteoporotic women: the link lies within the fat. Osteoporos Int 28:973–981. https://doi.org/10.1007/s00198-016-3792-3
Tamba S, Nishizawa H, Funahashi T, Okauchi Y, Ogawa T et al (2008) Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med 47:1175–1180. https://doi.org/10.2169/internalmedicine.47.0603
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R et al (2013) Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288:27138–27149. https://doi.org/10.1074/jbc.M113.485094
Chen W, Roncal-Jimenez C, Lanaspa M, Gerard S, Chonchol M et al (2014) Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism 63:150–160. https://doi.org/10.1016/j.metabol.2013.09.018
Fujita Y, Iki M, Tamaki J, Kouda K, Yura A et al (2013) Renal function and bone mineral density in community-dwelling elderly Japanese men: the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone 56:61–66. https://doi.org/10.1016/j.bone.2013.05.004
Acknowledgements
We would like to express our sincere gratitude to Zhen'ai Liang, who is studying in the Faculty of Humanities and Science of Wuhan University, for your care and encouragement during the drafting of this article.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2020YFF0304703).
Author information
Authors and Affiliations
Contributions
PL designed the current study and amended the paper. CM were the major writer of the paper, tested the entire index for all samples, and was responsible for the statistical analysis. RY created all the charts and provided suggestions for important intellectual content. CM, RY, JL, XW, JG, and EX performed the literature search and data collection. PL supervised the study and all authors discussed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was assessed and approved by Ethics Committee belonging to Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology (grant number: TJ-IRB20230108), which was implemented with Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ma, C., Yu, R., Li, J. et al. Association of serum uric acid levels with bone mineral density and the presence of osteoporosis in Chinese patients with Parkinson’s disease: a cross-sectional study. J Bone Miner Metab 41, 714–726 (2023). https://doi.org/10.1007/s00774-023-01446-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01446-7