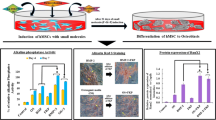
Abstract
Introduction
Tetracyclines (TCs) embrace a class of broad-spectrum antibiotics with unrelated effects at sub-antimicrobial levels, including an effective anti-inflammatory activity and stimulation of osteogenesis, allowing their repurposing for different clinical applications. Recently, sarecycline (SA)—a new-generation molecule with a narrower antimicrobial spectrum—was clinically approved due to its anti-inflammatory profile and reduced adverse effects verified with prolonged use. Notwithstanding, little is known about its osteogenic potential, previously verified for early generation TCs.
Materials and Methods
Accordingly, the present study is focused on the assessment of the response of human bone marrow-derived mesenchymal stromal cells (hBMSCs) to a concentration range of SA, addressing the metabolic activity, morphology and osteoblastic differentiation capability, further detailing the modulation of Wnt, Hedgehog, and Notch signaling pathways. In addition, an ex vivo organotypic bone development system was established in the presence of SA and characterized by microtomographic and histochemical analysis.
Results
hBMSCs cultured with SA presented a significantly increased metabolic activity compared to control, with an indistinguishable cell morphology. Moreover, RUNX2 expression was upregulated 2.5-fold, and ALP expression was increased around sevenfold in the presence of SA. Further, GLI2 expression was significantly upregulated, while HEY1 and HNF1A were downregulated, substantiating Hedgehog and Notch signaling pathways’ modulation. The ex vivo model developed in the presence of SA presented a significantly enhanced collagen deposition, extended migration areas of osteogenesis, and an increased bone mineral content, substantiating an increased osteogenic development.
Conclusion
Summarizing, SA is a promising candidate for drug repurposing within therapies envisaging the enhancement of bone healing/regeneration.







Similar content being viewed by others
References
Weinberg MA, Bral M (1998) Tetracycline and its analogues: a therapeutic paradigm in periodontal diseases. Crit Rev Oral Biol Med 9:322–332. https://doi.org/10.1177/10454411980090030501
Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352. https://doi.org/10.1111/bph.12139
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S et al (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. https://doi.org/10.1038/nrd.2018.168
Gomes PS, Fernandes MH (2007) Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch Oral Biol 52:251–259. https://doi.org/10.1016/j.archoralbio.2006.10.005
Muthukuru M, Sun J (2013) Doxycycline counteracts bone morphogenic protein 2-induced osteogenic mediators. J Periodontol 84:656–665. https://doi.org/10.1902/jop.2012.120338
Park J-B (2011) Effects of doxycycline, minocycline, and tetracycline on cell proliferation, differentiation, and protein expression in osteoprecursor cells. J Craniofac Surg 22:1839–1842. https://doi.org/10.1097/SCS.0b013e31822e8216
Suzuki A, Yagisawa J, Kumakura S, Tsutsui T (2006) Effects of minocycline and doxycycline on cell survival and gene expression in human gingival and periodontal ligament cells. J Periodontal Res 41:124–131. https://doi.org/10.1111/j.1600-0765.2005.00843.x
Lecio G, Ribeiro FV, Pimentel SP, Reis AA, da Silva RVC et al (2020) Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: randomized clinical, immune and microbiological trial. Clin Oral Investig 24:1269–1279. https://doi.org/10.1007/s00784-019-03005-9
Burgos RM, Rodvold KA (2019) Omadacycline: a novel aminomethylcycline. Infect Drug Resist 12:1895–1915. https://doi.org/10.2147/IDR.S171352
Haidari W, Bruinsma R, Cardenas-de-la-Garza JA, Feldman SR (2019) Sarecycline review. Ann Pharmacother 2019:106002801987311. https://doi.org/10.1177/1060028019873111
Zhanel G, Critchley I, Lin LY, Alvandi N (2019) Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother 63:1–15. https://doi.org/10.1128/AAC.01297-18
Bunick CG, Keri J, Tanaka SK, Furey N, Damiani G et al (2021) Antibacterial mechanisms and efficacy of sarecycline in animal models of infection and inflammation. Antibiotics 10:1–12. https://doi.org/10.3390/antibiotics10040439
Martin V, Garcia M, Montemor MF, Fernandes JCS, Gomes PS, Fernandes MH (2022) Simulating in vitro the bone healing potential of a degradable and tailored multifunctional mg-based alloy platform. Bioengineering 9:255. https://doi.org/10.3390/bioengineering9060255
Garbieri TF, Martin V, dos Santos CF, Gomes PDS, Fernandes MH (2021) The embryonic chick femur organotypic model as a tool to analyze the angiotensin ii axis on bone tissue. Pharmaceuticals 14:469. https://doi.org/10.3390/ph14050469
Araújo R, Martin V, Ferreira R, Fernandes MH, Gomes PS (2022) A new ex vivo model of the bone tissue response to the hyperglycemic environment–the embryonic chicken femur organotypic culture in high glucose conditions. Bone 158:116355. https://doi.org/10.1016/j.bone.2022.116355
Kanczler JM, Smith EL, Roberts CA, Oreffo ROC (2012) A Novel approach for studying the temporal modulation of embryonic skeletal development using organotypic bone cultures and microcomputed tomography. Tissue Eng Part C Methods 18:747–760. https://doi.org/10.1089/ten.tec.2012.0033
Ding DC, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transplant 20:5–14. https://doi.org/10.3727/096368910X
Shen LC, Chen YK, Lin LM, Shaw SY (2010) Anti-invasion and anti-tumor growth effect of doxycycline treatment for human oral squamous-cell carcinoma—in vitro and in vivo studies. Oral Oncol 46:178–184. https://doi.org/10.1016/j.oraloncology.2009.11.013
Onoda T, Ono T, Dhar DK, Yamanoi A, Fujii T, Nagasue N (2004) Doxycycline inhibits cell proliferation and invasive potential: combination therapy with cyclooxygenase-2 inhibitor in human colorectal cancer cells. J Lab Clin Med 143:207–216. https://doi.org/10.1016/j.lab.2003.12.012
Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) What is behind the non-antibiotic properties of minocycline? Pharmacol Res 67:18–30. https://doi.org/10.1016/j.phrs.2012.10.006
Sagar J, Sales K, Seifalian A, Winslet M (2010) Doxycycline in mitochondrial mediated pathway of apoptosis: a systematic review. Anticancer Agents Med Chem 10:556–563. https://doi.org/10.2174/187152010793498645
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB et al (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801. https://doi.org/10.1038/77528
Walters B, Uynuk-Ool T, Rothdiener M, Palm J, Hart ML et al (2017) Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-06794-9
Jhala D, Rather H, Vasita R (2016) Polycaprolactone-chitosan nanofibers influence cell morphology to induce early osteogenic differentiation. Biomater Sci 4:1584–1595. https://doi.org/10.1039/c6bm00492j
Almazin SM, Dziak R, Andreana S, Ciancio SG (2009) The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J Periodontol 80:999–1005. https://doi.org/10.1902/jop.2009.080574
Martin V, Ribeiro IA, Alves MM, Gonçalves L, Claudio RA et al (2019) Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater Sci Eng C 101:15–26. https://doi.org/10.1016/j.msec.2019.03.056
Kim Y, Kim J, Lee H, Shin W-R, Lee S et al (2019) Tetracycline analogs inhibit osteoclast differentiation by suppressing MMP-9-mediated histone H3 cleavage. Int J Mol Sci 20:4038. https://doi.org/10.3390/ijms20164038
Lattanzi W, Bernardini C (2012) Genes and molecular pathways of the osteogenic process. Osteogenesis. https://doi.org/10.5772/34022
Song L (2017) Calcium and bone metabolism indices, vol 82, 1st ed. Elsevier Inc., Hoboken. https://doi.org/10.1016/bs.acc.2017.06.005
Rosset EM, Bradshaw AD (2016) SPARC/osteonectin in mineralized tissue. Matrix Biol 52–54:78–87. https://doi.org/10.1016/j.matbio.2016.02.001
Hosseini S, Naderi-Manesh H, Vali H, Baghaban Eslaminejad M, Azam Sayahpour F et al (2019) Contribution of osteocalcin-mimetic peptide enhances osteogenic activity and extracellular matrix mineralization of human osteoblast-like cells. Colloids Surfaces B Biointerfaces 173:662–671. https://doi.org/10.1016/j.colsurfb.2018.10.035
Santibanez JF, Obradović H, Kukolj T, Krstić J (2018) Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev Dyn 247:382–395. https://doi.org/10.1002/dvdy.24554
Kuwahara ST, Liu S, Chareunsouk A, Serowoky M, Mariani FV (2022) On the horizon: Hedgehog signaling to heal broken bones. Bone Res 10:13. https://doi.org/10.1038/s41413-021-00184-8
Miao D, Liu H, Plut P, Niu M, Huo R et al (2004) Impaired endochondral bone development and osteopenia in Gli2-deficient mice. Exp Cell Res 294:210–222. https://doi.org/10.1016/j.yexcr.2003.10.021
Shimoyama A, Wada M, Ikeda F, Hata K, Matsubara T et al (2007) Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol Biol Cell 18:2411–2418. https://doi.org/10.1091/mbc.e06-08-0743
Bagheri L, Pellati A, Rizzo P, Aquila G, Massari L et al (2018) Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J Tissue Eng Regen Med 12:304–315. https://doi.org/10.1002/term.2455
Suh JH, Lee HW, Lee JW, Kim JB (2008) Hes1 stimulates transcriptional activity of Runx2 by increasing protein stabilization during osteoblast differentiation. Biochem Biophys Res Commun 367:97–102. https://doi.org/10.1016/j.bbrc.2007.12.100
Zamurovic N, Cappellen D, Rohner D, Susa M (2004) Coordinated activation of Notch, Wnt, and transforming growth factor-β signaling pathways in bone morphogenic protein 2-induced osteogenesis: Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J Biol Chem 279:37704–37715. https://doi.org/10.1074/jbc.M403813200
Qin Y, Zhang Q, Lee S, Zhong WL, Liu YR et al (2015) Doxycycline reverses epithelial-to-mesenchymal transition and suppresses the proliferation and metastasis of lung cancer cells. Oncotarget 6:4066779. https://doi.org/10.18632/oncotarget.5842
Bonner C, Farrelly AM, Concannon CG, Dussmann H, Baquie M et al (2011) Bone morphogenetic protein 3 controls insulin gene expression and is down-regulated in INS-1 cells inducibly expressing a hepatocyte nuclear factor 1A-maturity-onset diabetes of the young mutation. J Biol Chem 286:25719–25728. https://doi.org/10.1074/jbc.M110.215525
Di Pietro L, Barba M, Prampolini C, Ceccariglia S, Frassanito P et al (2020) Gli1 and axin2 are distinctive markers of human calvarial mesenchymal stromal cells in nonsyndromic craniosynostosis. Int J Mol Sci 21:1–19. https://doi.org/10.3390/ijms21124356
Smith E, Kanczler J, Oreffo R (2013) A new take on an old story: chick limb organ culture for skeletal niche development and regenerative medicine evaluation. Eur Cells Mater 26:91–106. https://doi.org/10.22203/eCM.v026a07
Cole AA, Chubinskaya S, Luchene LJ, Chlebek K, Orth MW et al (1994) Doxycycline disrupts chondrocyte differentiation and inhibits cartilage matrix degradation. Arthritis Rheum 37:1727–1734. https://doi.org/10.1002/art.1780371204
Tham AY, Gandhimathi C, Praveena J, Venugopal JR, Ramakrishna S, Kumar SD (2016) Minocycline loaded hybrid composites nanoparticles for mesenchymal stem cells differentiation into osteogenesis. Int J Mol Sci 17:1–16. https://doi.org/10.3390/ijms17081222
Gomes PS, Santos JD, Fernandes MH (2008) Cell-induced response by tetracyclines on human bone marrow colonized hydroxyapatite and Bonelike®. Acta Biomater 4:630–637. https://doi.org/10.1016/j.actbio.2007.12.006
Silva T, Silva JC, Colaco B, Gama A, Duarte-Araújo M et al (2018) In vivo tissue response and antibacterial efficacy of minocycline delivery system based on polymethylmethacrylate bone cement. J Biomater Appl 33:380–391. https://doi.org/10.1177/0885328218795290
Lee BS, Lee CC, Wang YP, Chen HJ, Lai CH et al (2016) Controlled-release of tetracycline and lovastatin by poly(D, L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int J Nanomed 11:285–297. https://doi.org/10.2147/IJN.S94270
Acknowledgements
The authors acknowledge the support of the i3S Scientific Platform Bioimaging, member of the PPBI (PPBI-POCI-01-0145-FEDER-022122), as well as HEMS—Histology and Electron Microscopy Department.
Funding
This work received financial support from national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through project 2022.06464.PTDC. Victor Martin thanks FCT for his PhD Grant Ref. 2020.04935.BD.
Author information
Authors and Affiliations
Contributions
VM: conceptualization, investigation, methodology, and writing—review & editing. LG: investigation and methodology. MHF: validation, and writing—review & editing. PG: conceptualization, validation, supervision, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest nor financial interests with Adooq Bioscience.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Martin, V., Grenho, L., Fernandes, M.H. et al. Repurposing sarecycline for osteoinductive therapies: an in vitro and ex vivo assessment. J Bone Miner Metab 41, 431–442 (2023). https://doi.org/10.1007/s00774-023-01428-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01428-9