Abstract
Introduction
Periprosthetic fracture caused by periprosthetic bone loss is an important concern in total hip arthroplasty (THA). Denosumab has been approved for postmenopausal women with osteoporosis who are at high risk of fracture. In this randomized controlled trial, we compared the effects of denosumab and risedronate on periprosthetic bone mineral density (BMD) after THA.
Materials and Methods
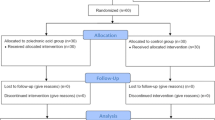
The current study analyzed 108 patients who were scheduled to have THA. For 2 years, the patients were randomly assigned to the following two treatment groups: denosumab (60 mg subcutaneously every 6 months) or risedronate (17.5 mg oral weekly). The BMD changes in all Gruen zones and bone turnover markers were measured at the 5th postoperative day (baseline) and 6, 12, 18, and 24 months postoperatively.
Results
The mean BMD in zones 1, 2, 6, and 7 was significantly higher with denosumab all administration at all postoperative time points compared to the risedronate group. The mean percentage changes in the BMD in these zones from baseline to 24 months postoperatively were + 11.9, + 2.9, + 8.1, and + 5.9% with denosumab group and − 9.6% –3.6, − 2.3, and − 19.2% with risedronate, respectively. The osteoclastic marker, tartrate-resistant acid phosphatase-5b (TRACP-5b), was significantly lower in the denosumab group compared to the risedronate group by 2 months.
Conclusion
Denosumab is more effective in preventing periprosthetic bone resorption than risedronate in the proximal femur. It also increased BMD around the stem implant following THA.






Similar content being viewed by others
References
Katz JN, Winter AR, Hawker G (2017) Measures of the appropriateness of elective orthopaedic joint and spine procedures. J Bone Joint Surg Am 99:e15
Springer BD, Etkin CD, Shores PB, Gioe TJ, Lewallen DG, Bozic KJ (2019) Perioperative periprosthetic femur fractures are strongly correlated with fixation method: an analysis from the american joint replacement registry. J Arthroplasty 34:5352–5354
Huang TW, Wang CJ, Shih HN, Chang Y, Huang KC, Peng KT, Lee MS (2017) Bone turnover and periprosthetic bone loss after cementless total hip arthroplasty can be restored by zoledronic acid: a prospective, randomized, open-label, controlled trial. BMC Musculoskelet Disord 18:209
Gao J, Gao C, Li H, Wang GS, Xu C, Ran J (2017) Effect of zoledronic acid on reducing femoral bone mineral density loss following total hip arthroplasty: A meta-analysis from randomized controlled trials. Int J Surg 47:116–126
Sköldenberg OG, Salemyr MO, Bodén HS, Ahl TE, Adolphson PY (2011) The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 93:1857–1864
Hailer NP, Garellick G, Karrholm J (2010) Uncemented and cemented primary total hip arthroplasty in the Swedich Hip Arthroplasty Register. Evaluation of 170,413 operations. Acta Orthop 81:34–41
Fujimoto T, Niimi A, Sawai T (1998) Effects of steroid-induced osteoporosis on osseointegration of titanium implant. Int J Oral Maxillofac Implants 13:183–189
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Ominsky MS, Jolette J, Smith SY, Vlasseros F, Samadfam R, Kostenuik PJ (2008) Transition from alendronate to denosumab resulted in further reductions in local and systemic bone turnover parameters and reduced cortical porosity in ovariectomized cyno- molgus monkeys. J Bone Miner Res 23:S61
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Tanaka H, Ikai T, Oh-hashi Y (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Gruen TA, McNeice GM, Amstutz HC (1979) ’Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 141:17–27
Yamasaki S, Masuhara K, Yamaguchi K, Nakai T, Fuji T, Seino Y (2007) Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty. Osteoporosis Int 18:1009–1015
Nystrom A, Kiritopoulos D, Ullmark G, Sorensen J, Petren-Mllmin M, Milbrink J, Hailer NP, Mallmin H (2020) Denosumab prevents early periprosthetic bone loss after uncemented total hip arthroplasty: results from a randomized placebo-controlled clinical trial. J Bone Miner Res 35:239–247
Augoulea A, Tsakonas E, Triantafyllopoulos I, Rizos D, Armeni E, Tsoltos N, Tournis S, Deligeoroglou E, Antoniou A, Lambrinoudaki I (2017) Comparative effects of denosumab or bisphosphonate treatment on bone mineral density and calcium metabolism in postmenopausal woman. J Musculoskelet Neur Interact. 17:444–449
Poole KE, Treece GM, Gee AH, Brown JP, McClung MR, Wang A, Libanati C (2015) Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res 30:46–54
Cristofolini L, Juszczyk M, Martelli S, Taddei F, Viceconti M (2007) In vitro replication of spontaneous fractures of the proximal human femur. J Biomech 40:2837–2845
Geusens P, Bevers MS, van Rietbergen B, Messina OD, Lespessailles E, Oliveri B, Chapurlat R, Engelke K, Chines A, Huang S, Saag KG, van den Bergh JP (2022) Effect of denosumab compared with risedronate on bone strength in patients initiating or continuing glucocorticoid treatment. J Bone Miner Res. Jun. 37:1136–1146
Wilkinson JM, Stockley I, Peel NF, Hamer AJ, Elson RA, Barrington NA, Eastell R (2001) Effect of pamidronate in preventing local bone loss after total hip arthroplasty: a randomized, double-blind, controlled trial. J Bone Miner Res 16:556–564
Eriksen EF, Keaveny TM, Gallagher ER, Krege JH (2014) Literature review: The effects of teriparatide therapy at the hip in patients with osteoporosiss. Bone 67:246–256
Kobayashi N, Inaba Y, Uchiyama M, Ike H, Kubota S, Saito T (2016) Teriparatide versus alendronate for the preservation of bone mineral density after total hip arthroplasty - a randomized controlled trial. J Arthroplasty 31:333–338
Syed KA, Kuzyk PR, Yoo DJ, Zdero R, Richards RR, Schemitsch EH (2013) Changes in femoral cortical porosity after reaming and intramedullary canal preparation in a canine model. J Arthroplasty 28:368–373
Bodén HS, Sköldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY (2006) Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA. Acta Orthop 77:877–885
Arabmotlagh M, Rittmeister M, Hennigs T (2006) Alendronate prevents femoral periprosthetic bone loss following total hip arthroplasty: prospective randomized double-blind study. J Orthop Res 24:1336–1341
Kiratli BJ, Checovich MM, McBeath AA, Wilson MA, Heiner JP (1996) Measurement of bone mineral density by dual-energy x-ray absorptiometry in patients with the Wisconsin hip, an uncemented femoral stem. J Arthroplasty 11:184–193
Yukizawa Y, Inaba Y, Kobayashi N, Choe H, Kubota S, Saito T (2017) Efficacy of alendronate for the prevention of bone loss in calcar region following total hip arthroplasty. J Arthroplasty Jul. 32:2176–2180
Muratore M, Quarta E, Quarta L, Calcagnile F, Grimaldi A, Orgiani MA, Marsilio A, Rollo G (2012) Ibandronate and cementless total hip arthroplasty: densitometric measurement of periprosthetic bone mass and new therapeutic approach to the prevention of aseptic loosening. Clin Cases Miner Bone Metab Jan. 9:50–55
Author information
Authors and Affiliations
Contributions
N.N. conceptualized the study and methodology and conducted the analysis and wrote the manuscript. K.H. supervised the study and reviewed and edited the manuscript. S.T. collected the data and provided conceptual advice. M.M. collected the data and provided conceptual advice.
Corresponding author
Ethics declarations
Conflict of interest
N.N. has no conflict of interest. K.H., S.T., and M.M serves as a consultant for Zimmer-Biomet Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakura, N., Hirakawa, K., Takayanagi, S. et al. Denosumab prevented periprosthetic bone resorption better than risedronate after total hip arthroplasty. J Bone Miner Metab 41, 239–247 (2023). https://doi.org/10.1007/s00774-023-01405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01405-2