Abstract
Introduction
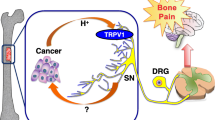
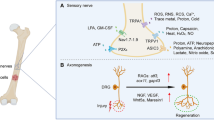
Cancer-induced bone pain (CIBP) is one of the most common and debilitating complications associated with bone metastasis. Although our understanding of the precise mechanism is limited, it has been known that bone is densely innervated, and that CIBP is elicited as a consequence of increased neurogenesis, reprogramming, and axonogenesis in conjunction with sensitization and excitation of sensory nerves (SNs) in response to the noxious stimuli that are derived from the tumor microenvironment developed in bone. Recent studies have shown that the sensitized and excited nerves innervating the tumor establish intimate communications with cancer cells by releasing various tumor-stimulating factors for tumor progression.
Approaches
In this review, the role of the interactions of cancer cells and SNs in bone in the pathophysiology of CIBP will be discussed with a special focus on the role of the noxious acidic tumor microenvironment, considering that bone is in nature hypoxic, which facilitates the generation of acidic conditions by cancer. Subsequently, the role of SNs in the regulation of cancer progression in the bone will be discussed together with our recent experimental findings.
Conclusion
It is suggested that SNs may be a newly-recognized important component of the bone microenvironment that contribute to not only in the pathophysiology of CIBP but also cancer progression in bone and dissemination from bone. Suppression of the activity of bone-innervating SNs, thus, may provide unique opportunities in the treatment of cancer progression and dissemination, as well as CIBP.


Similar content being viewed by others
Data availability statement
All the data presented in this article are available upon request to the corresponding author.
Abbreviations
- a3V-H + -ATPase:
-
A3 isoform V-H + -ATPase
- AN:
-
Autonomic nerve
- ASIC:
-
Acid-sensing ion channel
- CGRP:
-
Calcitonin gene-related protein
- CIBP:
-
Cancer-induced bone pain
- CNS:
-
Central nervous system
- CRPC:
-
Castration-resistant prostate cancer
- DRG:
-
Dorsal root ganglion
- GPR81:
-
G-protein-coupled receptor 81
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HMGB1:
-
High mobility group box 1
- HRPC:
-
Hormone-refractory prostate cancer
- IGF-1:
-
Insulin-like growth factor 1
- MCT1:
-
Monocarboxylate transporter 1
- MCT4:
-
Monocarboxylate transporter 4
- NGF:
-
Nerve growth factor
- NMDA:
-
N-methyl-D-aspartate
- pCREB:
-
Phosphorylated cyclic AMP-responsive element-binding protein
- pERK1/2:
-
Phosphorylated extracellular receptor kinase 1/2
- PNI:
-
Perineural invasion
- PTH-rP:
-
Parathyroid hormone-related protein
- RAGE:
-
Receptor for advanced glycation end products
- SN:
-
Sensory nerve
- SRE:
-
Skeletal-related events
- TGFβ1:
-
Transforming growth factor β1
- TLR:
-
Toll-like receptors
- TRPV1:
-
Transient receptor potential channel, vanilloid subfamily member 1
- WT:
-
Wild-type
References
Goldberg DS, McGee SJ (2011) Pain as a global public health priority. BMC Public Health 11:770. https://doi.org/10.1186/1471-2458-11-770
Swieboda P, Filip R, Prystupa A, Drozd M (2013) Assessment of pain: types, mechanism and treatment. Ann Agric Environ Med Spec 1:2–7
Falk S, Dickenson AH (2014) Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 32:1647–1654. https://doi.org/10.1200/jco.2013.51.7219
Zylla D, Steele G, Gupta P (2017) A systematic review of the impact of pain on overall survival in patients with cancer. Support Care Cancer 25:1687–1698. https://doi.org/10.1007/s00520-017-3614-y
Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R, Costa L (2020) Bone metastases. Nat Rev Dis Primers 6:83. https://doi.org/10.1038/s41572-020-00216-3
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletalmorbidity. Clin Cancer Res 12:6243s-s6249. https://doi.org/10.1158/1078-0432.Ccr-06-0931
Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, Patrick DL, Clemons M, Tonkin K, Masuda N, Lipton A, de Boer R, Salvagni S, Oliveira CT, Qian Y, Jiang Q, Dansey R, Braun A, Chung K (2013) Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer 119:832–838. https://doi.org/10.1002/cncr.27789
Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, Confavreux CB, Holen I (2021) Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev 101:797–855. https://doi.org/10.1152/physrev.00012.2019
von Moos R, Costa L, Ripamonti CI, Niepel D, Santini D (2017) Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer 71:80–94. https://doi.org/10.1016/j.ejca.2016.10.021
Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ (2008) Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 26:2544–2549. https://doi.org/10.1200/jco.2007.15.0367
Coveler AL, Mizrahi J, Eastman B, Apisarnthanarax SJ, Dalal S, McNearney T, Pant S (2021) Pancreas cancer-associated pain management. Oncologist 26:e971–e982. https://doi.org/10.1002/onco.13796
Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, Link MP, Lustig C, McLaughlin J, Ried LD, Turrisi AT 3rd, Unützer J, Vernon SW (2003) National institutes of health state-of-the-science conference statement: symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst 95:1110–1117. https://doi.org/10.1093/jnci/djg014
Staats PS, Hekmat H, Sauter P, Lillemoe K (2001) The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain Med 2:28–34. https://doi.org/10.1046/j.1526-4637.2001.002001028.x
Mantyh PW (2014) Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 8:83–90. https://doi.org/10.1097/spc.0000000000000048
Ivanusic JJ (2017) Molecular mechanisms that contribute to bone marrow pain. Front Neurol 8:458. https://doi.org/10.3389/fneur.2017.00458
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115:3379–3391. https://doi.org/10.1002/cncr.24396
Bapat AA, Hostetter G, Von Hoff DD, Han H (2011) Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer 11:695–707. https://doi.org/10.1038/nrc3131
Ivanusic JJ (2009) Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol 517:276–283. https://doi.org/10.1002/cne.22160
Jung WC, Levesque JP, Ruitenberg MJ (2017) It takes nerve to fight back: the significance of neural innervation of the bone marrow and spleen for immune function. Semin Cell Dev Biol 61:60–70. https://doi.org/10.1016/j.semcdb.2016.08.010
Brazill JM, Beeve AT, Craft CS, Ivanusic JJ, Scheller EL (2019) Nerves in bone: evolving concepts in pain and anabolism. J Bone Miner Res 34:1393–1406. https://doi.org/10.1002/jbmr.3822
Wan QQ, Qin WP, Ma YX, Shen MJ, Li J, Zhang ZB, Chen JH, Tay FR, Niu LN, Jiao K (2021) Crosstalk between Bone and Nerves within Bone. Adv Sci (Weinh) 8:2003390. https://doi.org/10.1002/advs.202003390
Cooper RR (1968) Nerves in cortical bone. Science 160:327–328. https://doi.org/10.1126/science.160.3825.327
Serre CM, Farlay D, Delmas PD, Chenu C (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623–629. https://doi.org/10.1016/s8756-3282(99)00215-x
Irie K, Hara-Irie F, Ozawa H, Yajima T (2002) Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech 58:85–90. https://doi.org/10.1002/jemt.10122
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW (2002) Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113:155–166. https://doi.org/10.1016/s0306-4522(02)00165-3
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S et al (2013) Sema3A regulates bone-mass accrual through sensory innervations. Nature 497:490–493. https://doi.org/10.1038/nature12115
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413:203–210. https://doi.org/10.1038/35093019
Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW (2018) The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience 387:178–190. https://doi.org/10.1016/j.neuroscience.2018.01.047
Hiasa M, Okui T, Allette YM, Ripsch MS, Sun-Wada GH, Wakabayashi H, Roodman GD, White FA, Yoneda T (2017) Bone pain induced by multiple myeloma is reduced by targeting V-ATPase and ASIC3. Cancer Res 77:1283–1295. https://doi.org/10.1158/0008-5472.Can-15-3545
Wakabayashi H, Wakisaka S, Hiraga T, Hata K, Nishimura R, Tominaga M, Yoneda T (2018) Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J Bone Miner Metab 36:274–285. https://doi.org/10.1007/s00774-017-0842-7
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341:1236361. https://doi.org/10.1126/science.1236361
Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H (2015) Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res 75:1777–1781. https://doi.org/10.1158/0008-5472.Can-14-3180
Elefteriou F (2018) Impact of the autonomic nervous system on the skeleton. Physiol Rev 98:1083–1112. https://doi.org/10.1152/physrev.00014.2017
Zahalka AH, Frenette PS (2020) Nerves in cancer. Nat Rev Cancer 20:143–157. https://doi.org/10.1038/s41568-019-0237-2
Lorenz MR, Brazill JM, Beeve AT, Shen I, Scheller EL (2021) A neuroskeletal atlas: spatial mapping and contextualization of axon subtypes innervating the long bones of C3H and B6 mice. J Bone Miner Res 36:1012–1025. https://doi.org/10.1002/jbmr.4273
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69:1–18. https://doi.org/10.1016/s0304-3959(96)03267-8
Zajączkowska R, Kocot-Kępska M, Leppert W, Wordliczek J (2019) Bone pain in cancer patients: mechanisms and current treatment. Int J Mol Sci. https://doi.org/10.3390/ijms20236047
Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M (2021) Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res 81:1431–1440. https://doi.org/10.1158/0008-5472.Can-20-2793
Okui T, Hiasa M, Ryumon S, Ono K, Kunisada Y, Ibaragi S, Sasaki A, Roodman GD, White FA, Yoneda T (2021) The HMGB1/RAGE axis induces bone pain associated with colonization of 4T1 mouse breast cancer in bone. J Bone Oncol 26:100330. https://doi.org/10.1016/j.jbo.2020.100330
Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW (2010) Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci 30:14649–14656. https://doi.org/10.1523/jneurosci.3300-10.2010
March B, Faulkner S, Jobling P, Steigler A, Blatt A, Denham J, Hondermarck H (2020) Tumour innervation and neurosignalling in prostate cancer. Nat Rev Urol 17:119–130. https://doi.org/10.1038/s41585-019-0274-3
Maes C, Carmeliet G, Schipani E (2012) Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 8:358–366. https://doi.org/10.1038/nrrheum.2012.36
Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9:285–296. https://doi.org/10.1038/nrm2354
Liberti MV, Locasale JW (2016) The Warburg Effect: how does it benefit cancer cells? Trends Biochem Sci 41:211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Parks SK, Mueller-Klieser W, Pouysségur J (2020) Lactate and Acidity in the cancer microenvironment. Ann Rev Cancer Biol 4:141–158. https://doi.org/10.1146/annurev-cancerbio-030419-033556
Nakanishi-Matsui M, Matsumoto N (2022) V-ATPase a3 subunit in secretory lysosome trafficking in osteoclasts. Biol Pharm Bull 45:1426–1431. https://doi.org/10.1248/bpb.b22-00371
Maeda H, Kowada T, Kikuta J, Furuya M, Shirazaki M, Mizukami S, Ishii M, Kikuchi K (2016) Real-time intravital imaging of pH variation associated with osteoclast activity. Nat Chem Biol 12:579–585. https://doi.org/10.1038/nchembio.2096
Mantyh PW (2006) Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 7:797–809. https://doi.org/10.1038/nrn1914
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. https://doi.org/10.1016/j.cell.2009.09.028
Sun WH, Dai SP (2018) Tackling pain associated with rheumatoid arthritis: proton-sensing receptors. Adv Exp Med Biol 1099:49–64. https://doi.org/10.1007/978-981-13-1756-9_5
Louca Jounger S, Eriksson N, Lindskog H, Oscarsson A, Simonsson V, Ernberg M, Christidis N (2019) Repeated buffered acidic saline infusion in the human masseter muscle as a putative experimental pain model. Sci Rep 9:15474. https://doi.org/10.1038/s41598-019-51670-3
Esposito MF, Malayil R, Hanes M, Deer T (2019) Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med 20:S23-s30. https://doi.org/10.1093/pm/pnz012
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Bujak JK, Kosmala D, Szopa IM, Majchrzak K, Bednarczyk P (2019) Inflammation, cancer and immunity-implication of TRPV1 channel. Front Oncol 9:1087. https://doi.org/10.3389/fonc.2019.01087
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313. https://doi.org/10.1126/science.288.5464.306
Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW (2014) Calcium-permeable ion channels in pain signaling. Physiol Rev 94:81–140. https://doi.org/10.1152/physrev.00023.2013
Lieben L, Carmeliet G (2012) The involvement of TRP channels in Bone homeostasis. Front Endocrinol (Lausanne) 3:99. https://doi.org/10.3389/fendo.2012.00099
Li L, Chen C, Chiang C, Xiao T, Chen Y, Zhao Y, Zheng D (2021) The impact of TRPV1 on cancer pathogenesis and therapy: a systematic review. Int J Biol Sci 17:2034–2049. https://doi.org/10.7150/ijbs.59918
Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW (2005) Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 25:3126–3131. https://doi.org/10.1523/jneurosci.3815-04.2005
Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A (2007) Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 148:560–572. https://doi.org/10.1016/j.neuroscience.2007.05.049
Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A (2009) SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth 102:251–258. https://doi.org/10.1093/bja/aen347
Xu Q, Zhang XM, Duan KZ, Gu XY, Han M, Liu BL, Zhao ZQ, Zhang YQ (2013) Peripheral TGF-β1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci 33:19099–19111. https://doi.org/10.1523/jneurosci.4852-12.2013
Li Y, Cai J, Han Y, Xiao X, Meng XL, Su L, Liu FY, Xing GG, Wan Y (2014) Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain 18:774–784. https://doi.org/10.1002/j.1532-2149.2013.00420.x
Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG (2015) Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain 156:1124–1144. https://doi.org/10.1097/j.pain.0000000000000158
Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T (2006) Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 39:1107–1115. https://doi.org/10.1016/j.bone.2006.04.033
Shepherd AJ, Mickle AD, Kadunganattil S, Hu H, Mohapatra DP (2018) Parathyroid hormone-related peptide elicits peripheral TRPV1-dependent mechanical hypersensitivity. Front Cell Neurosci 12:38. https://doi.org/10.3389/fncel.2018.00038
Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A (2014) TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 157:1023–1036. https://doi.org/10.1016/j.cell.2014.03.051
Lee CH, Chen CC (2018) Roles of ASICs in nociception and proprioception. Adv Exp Med Biol 1099:37–47. https://doi.org/10.1007/978-981-13-1756-9_4
Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R (1998) An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. NeuroReport 9:1109–1113. https://doi.org/10.1097/00001756-199804200-00028
Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP (2005) Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun 337:349–354. https://doi.org/10.1016/j.bbrc.2005.09.054
Wemmie JA, Taugher RJ, Kreple CJ (2013) Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 14:461–471. https://doi.org/10.1038/nrn3529
Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106:229–239. https://doi.org/10.1016/s0304-3959(03)00269-0
Wu WL, Cheng CF, Sun WH, Wong CW, Chen CC (2012) Targeting ASIC3 for pain, anxiety, and insulin resistance. Pharmacol Ther 134:127–138. https://doi.org/10.1016/j.pharmthera.2011.12.009
Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO (2010) Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 161:950–960. https://doi.org/10.1111/j.1476-5381.2010.00918.x
Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL (2010) A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68:61–72. https://doi.org/10.1016/j.neuron.2010.09.001
Hsieh WS, Kung CC, Huang SL, Lin SC, Sun WH (2017) TDAG8, TRPV1, and ASIC3 involved in establishing hyperalgesic priming in experimental rheumatoid arthritis. Sci Rep 7:8870. https://doi.org/10.1038/s41598-017-09200-6
Nagae M, Hiraga T, Yoneda T (2007) Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 25:99–104. https://doi.org/10.1007/s00774-006-0734-8
Qiu F, Wei X, Zhang S, Yuan W, Mi W (2014) Increased expression of acid-sensing ion channel 3 within dorsal root ganglia in a rat model of bone cancer pain. NeuroReport 25:887–893. https://doi.org/10.1097/wnr.0000000000000182
Qian HY, Zhou F, Wu R, Cao XJ, Zhu T, Yuan HD, Chen YN, Zhang PA (2021) Metformin attenuates bone cancer pain by reducing TRPV1 and ASIC3 expression. Front Pharmacol 12:713944. https://doi.org/10.3389/fphar.2021.713944
Terpos E, Zamagni E, Lentzsch S, Drake MT, García-Sanz R, Abildgaard N, Ntanasis-Stathopoulos I, Schjesvold F, de la Rubia J, Kyriakou C, Hillengass J, Zweegman S, Cavo M, Moreau P, San-Miguel J, Dimopoulos MA, Munshi N, Durie BGM, Raje N (2021) Treatment of multiple myeloma-related bone disease: recommendations from the bone working group of the international myeloma working group. Lancet Oncol 22:e119–e130. https://doi.org/10.1016/s1470-2045(20)30559-3
Mamet J, Baron A, Lazdunski M, Voilley N (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22:10662–10670. https://doi.org/10.1523/jneurosci.22-24-10662.2002
Marra S, Ferru-Clément R, Breuil V, Delaunay A, Christin M, Friend V, Sebille S, Cognard C, Ferreira T, Roux C, Euller-Ziegler L, Noel J, Lingueglia E, Deval E (2016) Non-acidic activation of pain-related acid-sensing ion channel 3 by lipids. Embo J 35:414–428. https://doi.org/10.15252/embj.201592335
Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D (2013) HMGB1 in cancer: good, bad, or both? Clin Cancer Res 19:4046–4057. https://doi.org/10.1158/1078-0432.Ccr-13-0495
Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, Watkins LR, Wilson IA, Yin H (2016) HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep 17:1128–1140. https://doi.org/10.1016/j.celrep.2016.09.076
Sun S, Li H, Chen J, Qian Q (2017) Lactic acid: no longer an inert and end-product of glycolysis. Physiology (Bethesda) 32:453–463. https://doi.org/10.1152/physiol.00016.2017
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123:3685–3692. https://doi.org/10.1172/jci69741
Brooks GA (2018) The science and translation of lactate shuttle theory. Cell Metab 27:757–785. https://doi.org/10.1016/j.cmet.2018.03.008
Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 19:235–249. https://doi.org/10.1038/nrn.2018.19
Brown TP, Ganapathy V (2020) Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther 206:107451. https://doi.org/10.1016/j.pharmthera.2019.107451
Ishihara S, Hata K, Hirose K, Okui T, Toyosawa S, Uzawa N, Nishimura R, Yoneda T (2022) The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer. Sci Rep 12:6261. https://doi.org/10.1038/s41598-022-10143-w
Simatou A, Simatos G, Goulielmaki M, Spandidos DA, Baliou S, Zoumpourlis V (2020) Historical retrospective of the SRC oncogene and new perspectives (review). Mol Clin Oncol 13:21. https://doi.org/10.3892/mco.2020.2091
Li Y, Bao Y, Zheng H, Qin Y, Hua B (2021) The nonreceptor protein tyrosine kinase Src participates in every step of cancer-induced bone pain. Biomed Pharmacother 141:111822. https://doi.org/10.1016/j.biopha.2021.111822
De Felice M, Lambert D, Holen I, Escott KJ, Andrew D (2016) Effects of Src-kinase inhibition in cancer-induced bone pain. Mol Pain. https://doi.org/10.1177/1744806916643725
Appel CK, Gallego-Pedersen S, Andersen L, Blancheflor Kristensen S, Ding M, Falk S, Sayilekshmy M, Gabel-Jensen C, Heegaard AM (2017) The Src family kinase inhibitor dasatinib delays pain-related behaviour and conserves bone in a rat model of cancer-induced bone pain. Sci Rep 7:4792. https://doi.org/10.1038/s41598-017-05029-1
Danson S, Mulvey MR, Turner L, Horsman J, Escott K, Coleman RE, Ahmedzai SH, Bennett MI, Andrew D (2019) An exploratory randomized-controlled trial of the efficacy of the Src-kinase inhibitor saracatinib as a novel analgesic for cancer-induced bone pain. J Bone Oncol 19:100261. https://doi.org/10.1016/j.jbo.2019.100261
Oudard S, Banu E, Medioni J, Scotte F, Banu A, Levy E, Wasserman J, Kacso G, Andrieu JM (2009) What is the real impact of bone pain on survival in patients with metastatic hormone-refractory prostate cancer treated with docetaxel? BJU Int 103:1641–1646. https://doi.org/10.1111/j.1464-410X.2008.08283.x
Inoue T, Segawa T, Kamba T, Yoshimura K, Nakamura E, Nishiyama H, Ito N, Kamoto T, Habuchi T, Ogawa O (2009) Prevalence of skeletal complications and their impact on survival of hormone refractory prostate cancer patients in Japan. Urology 73:1104–1109. https://doi.org/10.1016/j.urology.2008.07.062
Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, Lévy J, Miller K, Nilsson S, Petrenciuc O, Tucci M, Wirth M, Federhofer J, O’Sullivan JM (2016) Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 17:1306–1316. https://doi.org/10.1016/s1470-2045(16)30173-5
Koizumi M, Yoshimoto M, Kasumi F, Iwase T, Ogata E (2010) Post-operative breast cancer patients diagnosed with skeletal metastasis without bone pain had fewer skeletal-related events and deaths than those with bone pain. BMC Cancer 10:423. https://doi.org/10.1186/1471-2407-10-423
Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ (2019) Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res 9:1–21
Delahunt B, Murray JD, Steigler A, Atkinson C, Christie D, Duchesne G, Egevad L, Joseph D, Matthews J, Oldmeadow C, Samaratunga H, Spry NA, Srigley JR, Hondermarck H, Denham JW (2020) Perineural invasion by prostate adenocarcinoma in needle biopsies predicts bone metastasis: Ten year data from the TROG 03.04 RADAR Trial. Histopathology 77:284–292. https://doi.org/10.1111/his.14107
Duraker N, Caynak ZC, Türköz K (2006) Perineural invasion has no prognostic value in patients with invasive breast carcinoma. Breast 15:629–634. https://doi.org/10.1016/j.breast.2005.12.003
Karak SG, Quatrano N, Buckley J, Ricci A Jr (2010) Prevalence and significance of perineural invasion in invasive breast carcinoma. Conn Med 74:17–21
Gobbi H, Jensen RA, Simpson JF, Olson SJ, Page DL (2001) Atypical ductal hyperplasia and ductal carcinoma in situ of the breast associated with perineural invasion. Hum Pathol 32:785–790. https://doi.org/10.1053/hupa.2001.27637
Wang X, Lan H, Shen T, Gu P, Guo F, Lin X, Jin K (2015) Perineural invasion: a potential reason of hepatocellular carcinoma bone metastasis. Int J Clin Exp Med 8:5839–5846
Liu F, Zhao J, Xie J, Xie L, Zhu J, Cai S, Zheng H, Xu Y (2016) Prognostic risk factors in patients with bone metastasis from colorectal cancer. Tumour Biol. https://doi.org/10.1007/s13277-016-5465-4
Michalek J, Brychtova S, Pink R, Dvorak Z (2019) Prognostic and predictive markers for perineural and bone invasion of oral squamous cell carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 163:302–308. https://doi.org/10.5507/bp.2019.032
Braun S, Vogl FD, Naume B, Janni W, Osborne MP et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802. https://doi.org/10.1056/NEJMoa050434
Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Jückstock J, Borgen E, Rack B, Braun S, Sommer H, Solomayer E, Pantel K, Nesland J, Friese K, Naume B (2011) Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse–a European pooled analysis. Clin Cancer Res 17:2967–2976. https://doi.org/10.1158/1078-0432.Ccr-10-2515
Giordano A, Gao H, Cohen EN, Anfossi S, Khoury J, Hess K, Krishnamurthy S, Tin S, Cristofanilli M, Hortobagyi GN, Woodward WA, Lucci A, Reuben JM (2013) Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann Oncol 24:2515–2521. https://doi.org/10.1093/annonc/mdt223
Yoneda T, Hiasa M, Okui T, Hata K (2021) Sensory nerves: a driver of the vicious cycle in bone metastasis? J Bone Oncol 30:100387. https://doi.org/10.1016/j.jbo.2021.100387
Mogil JS (2019) The translatability of pain across species. Philos Trans R Soc Lond B Biol Sci 374:20190286. https://doi.org/10.1098/rstb.2019.0286
Iftinca M, Defaye M, Altier C (2021) TRPV1-targeted drugs in development for human pain conditions. Drugs 81:7–27. https://doi.org/10.1007/s40265-020-01429-2
Funding
This study was supported by Japan Society for the Promotion of Science, 20H03859, Toshiyuki Yoneda. This study is supported by the Project Development Team within the ICTSI NIH/NCRR (#TR000006), the IU Health Strategic Research Initiative in Oncology, and start-up fund of Indiana University School of Medicine for TY, Japan Society for the Promotion of Science Grants-in-aid for Research Activity Start-up and Postdoctoral Fellowship for Research Abroad to HM and TO, the Grants-in-Aid for Young Scientists (JSPS KAKENHI grant no. 18K17225) to TO, and the Grants-in-Aid for Scientific Research (JSPS KAKENHI grant no. 17H04377) to TY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoneda, T., Hiasa, M., Okui, T. et al. Cancer–nerve interplay in cancer progression and cancer-induced bone pain. J Bone Miner Metab 41, 415–427 (2023). https://doi.org/10.1007/s00774-023-01401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-023-01401-6