Abstract
Introduction
Evidence on second-line agents for osteoporosis and osteopenia associated with glucocorticoid use after first-line bisphosphonate therapy is limited. We, therefore, examine the efficacy of denosumab on bisphosphonate-treated osteoporosis and osteopenia in Japanese systemic rheumatic disease (SRD) patients receiving glucocorticoids.
Materials and methods
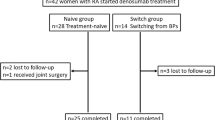
Glucocorticoid-treated SRD patients with a pre-existing fragility fracture, either lumbar spine (LS) or femoral neck (FN) bone mineral density (BMD) T-score of ≤ −2.5 or of ≤ −1.5 without a significant increase in BMD in the past year despite oral bisphosphonate therapy were enrolled in this study. They were randomized to switch to 60 mg subcutaneous denosumab every six months (switching group) or to continue the bisphosphonate (continuing group). The primary endpoint was the percent change from baseline in BMD at the LS and FN at week 52.
Results
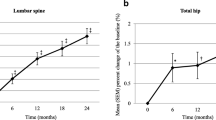
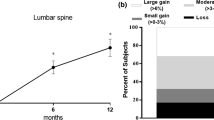
Of the 39 subjects, 19 were assigned to the switching group and 20 to the continuing group. The switching group showed significant increases in LS BMD (5.7% vs. 1.1%, p = 0.002) and FN BMD (4.2% vs. −0.3%, p = 0.008) at week 52 than the continuing group, with a significant decrease in serum tartrate-resistant acid phosphatase 5b (−28.1% vs. 7.0%, p < 0.001) and improved patient satisfaction.
Conclusion
Switching to denosumab demonstrated greater efficacy than continuing bisphosphonates in increasing BMD, inhibiting osteoclast activation, and enhancing patient satisfaction in Japanese bisphosphonate-treated osteoporosis and osteopenia patients with concomitant SRD receiving glucocorticoids.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, T.N., upon reasonable request.
References
Curtis JR, Saag KG (2007) Prevention and treatment of glucocorticoid-induced osteoporosis. Curr Osteoporos Rep 5:14–21. https://doi.org/10.1007/BF02938618
Van Staa TP (2006) The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 79:129–137. https://doi.org/10.1007/s00223-006-0019-1
Lems WF (2007) Bisphosphonates and glucocorticoids: effects on bone quality. Arthritis Rheum 56:3518–3522. https://doi.org/10.1002/art.22975
Saag KG (2003) Glucocorticoid-induced osteoporosis. Endocrinol Metab Clin North Am 32:135–157. https://doi.org/10.1016/s0889-8529(02)00064-6
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000. https://doi.org/10.1359/jbmr.2000.15.6.993
Vestergaard P, Olsen ML, Paaske Johnsen S, Rejnmark L, Sørensen HT, Mosekilde L (2003) Corticosteroid use and risk of hip fracture: a population-based case-control study in Denmark. J Intern Med 254:486–493. https://doi.org/10.1046/j.1365-2796.2003.01219.x
Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, Kovac SH, Spettell CM, Saag KG (2006) Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 55:420–426. https://doi.org/10.1002/art.21984
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, Giorgino R, Moro L, Giustina A (2006) High prevalence of asymptomatic vertebral fractures in postmenopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39:253–259. https://doi.org/10.1016/j.bone.2006.02.005
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J et al (2017) American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 69:1095–1110. https://doi.org/10.1002/acr.23279
Suzuki Y, Nawata H, Soen S, Fujiwara S, Nakayama H, Tanaka I, Ozono K, Sagawa A, Takayanagi R, Tanaka H, Miki T, Masunari N, Tanaka Y (2014) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32:337–350. https://doi.org/10.1007/s00774-014-0586-6
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–2276. https://doi.org/10.1007/s00198-012-1958-1
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Chapurlat R, Wang A, Pannacciulli N, Lems WF (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, activecontrolled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6:445–454. https://doi.org/10.1016/S2213-8587(18)30075-5
Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, Emkey R, Butler PW, Yin X, Lems WF (2019) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four–month randomized, double-blind, double-dummy trial. Arthritis Rheumatol 71:1174–1184. https://doi.org/10.1002/art.40874
Mok CC, Ho LY, Ma KM (2015) Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone 75:222–228. https://doi.org/10.1016/j.bone.2015.03.002
Suzuki T, Nakamura Y, Kato H (2018) Significant improvement of bone mineral density by denosumab without bisphosphonate pre-treatment in glucocorticoid-induced osteoporosis. Mod Rheumatol 28:885–889. https://doi.org/10.1080/14397595.2017.1416919
Hirooka Y, Nozaki Y, Inoue A, Li J, Shiga T, Kishimoto K, Sugiyama M, Kinoshita K, Funauchi M, Matsumura I (2020) Effects of denosumab versus teriparatide in glucocorticoid-induced osteoporosis patients with prior bisphosphonate treatment. Bone Rep 13:100293. https://doi.org/10.1016/j.bonr.2020.100293
Tamechika SY, Sasaki K, Hayami Y, Ohmura SI, Maeda S, Iwagaitsu S, Naniwa T (2018) Patient satisfaction and efficacy of switching from weekly bisphosphonates to monthly minodronate for treatment and prevention of glucocorticoid-induced osteoporosis in Japanese patients with systemic rheumatic diseases: a randomized, clinical trial. Arch Osteoporos 13:67. https://doi.org/10.1007/s11657-018-0451-7
Furuta K, Adachi K, Arima N, Tanaka S, Miki M, Azumi T, Koshino K, Kinoshita Y (2008) Study on the recognition of Japanese adults for upper abdominal symptoms (in Japanese). Nippon Shokakibyo Gakkai Zasshi 105:817–824
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81. https://doi.org/10.1359/jbmr.090716
R Core Team. R (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
National Cancer Institute (2020) Common terminology criteria for adverse events, version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf 2017
Matsuno H (2016) Assessment of distal radius bone mineral density in osteoporosis patients receiving denosumab, including those with rheumatoid arthritis and those receiving oral glucocorticoids. Drugs R D 16:347–353. https://doi.org/10.1007/s40268-016-0146-8
Iseri K, Iyoda M, Watanabe M, Matsumoto K, Sanada D, Inoue T, Tachibana S, Shibata T (2018) The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial. PLoS ONE 13:e0193846. https://doi.org/10.1371/journal.pone.0193846
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures–a randomized, active comparator, double-blind study. Bone 49:605–612. https://doi.org/10.1016/j.bone.2011.07.011
Matsumoto T, Yamamoto K, Takeuchi T, Tanaka Y, Tanaka S, Nakano T, Ito M, Tomomitsu T, Hirakawa A, Soen S (2020) Eldecalcitol is superior to alfacalcidol in maintaining bone mineral density in glucocorticoid-induced osteoporosis patients (e-GLORIA). J Bone Miner Metab 38:522–532. https://doi.org/10.1007/s00774-020-01091-4
Ebina K, Kashii M, Hirao M, Hashimoto J, Noguchi T, Koizumi K, Kitaguchi K, Matsuoka H, Iwahashi T, Tsukamoto Y, Yoshikawa H (2017) Comparison of the effects of denosumab between a native vitamin D combination and an active vitamin D combination in patients with postmenopausal osteoporosis. J Bone Miner Metab 35:571–580. https://doi.org/10.1007/s00774-016-0792-5
Cosman F, Kendler DL, Langdahl BL, Leder BZ, Lewiecki EM, Miyauchi A, Rojeski M, McDermott M, Oates MK, Milmont CE, Libanati C, Ferrari S (2022) Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int 33:1243–1256. https://doi.org/10.1007/s00198-021-06174-0
Morizio P, Burkhart JI, Ozawa S (2018) Denosumab: a unique perspective on adherence and cost-effectiveness compared with oral bisphosphonates in osteoporosis patients. Ann Pharmacother 52:1031–1041. https://doi.org/10.1177/1060028018768808
Koller G, Goetz V, Vandermeer B, Homik J, McAlister FA, Kendler D, Ye C (2020) Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int 31:2093–2102. https://doi.org/10.1007/s00198-020-05507-9
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938. https://doi.org/10.1001/jama.296.24.2927
Acknowledgements
The authors would like to thank Drs. Shiho Iwagaitsu, Department of Nephrology and Rheumatology, Aichi Medical University Hospital, who cared for the patients, and Prof. Akio Niimi, Department of Respiratory Medicine, Allergy and Clinical Immunology, Nagoya City University Graduate School of Medical Sciences, who provided general support as a department chair.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
Study design: SO and TN. Study conduct and data collection: SO, ST, and TN. Data analysis: ST and TN. Data interpretation: ST and TN. The first draft of the manuscript: ST and TN. Revising manuscript content: ST and TN with assistance in the form of critique and suggestions from all authors. Approving final version of manuscript: all authors. TN takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
T.N. received grants from Chugai Pharmaceutical Co., Ltd, speaker fees from Chugai Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd, Eisai Co., Ltd, and Astellas Pharma Inc. S.T. received speaker fees from Chugai Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd, Eisai Co., Ltd, Daiichi-Sankyo Co., Ltd., and Astellas Pharma Inc. S.O. received speaker fees from Chugai Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd, Daiichi-Sankyo Co., Ltd., and Astellas Pharma Inc. S.M. received speaker fees from Ono Pharmaceutical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tamechika, Sy., Ohmura, Si., Maeda, S. et al. Efficacy of denosumab on bisphosphonate-treated osteoporosis and osteopenia in systemic rheumatic disease patients receiving glucocorticoids. J Bone Miner Metab 41, 203–211 (2023). https://doi.org/10.1007/s00774-022-01393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01393-9