Abstract
Introduction
Heterotopic ossification of tendons and ligaments is a painful and debilitating disease with no effective treatment. Although aging has been reported to be correlated with the occurrence and development of this disease, the mechanism remains unknown.
Materials and methods
In the present study, we generated Bmal1-/- mice, which disrupted the circadian clock and displayed premature aging, as an aging model to explore the role of Bmal1 in TGF-beta (β)/BMP signaling in progressive heterotopic ossification of tendons and ligaments with aging.
Results
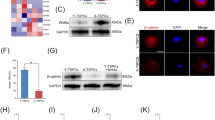
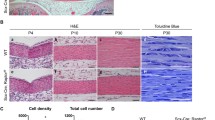
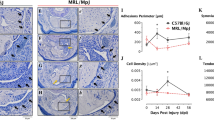
We first confirmed that BMAL1 expression is downregulated in human fibroblasts from ossification of the posterior longitudinal ligament using online datasets. Bmal1 deficiency in mice caused significantly progressive heterotopic ossification with aging starting at week 6, notably in the Achilles tendons and posterior longitudinal ligaments. Ossification of the Achilles tendons was accompanied by progressive motor dysfunction of the ankle joint. Histology and immunostaining showed markedly increased endochondral ossification in the posterior longitudinal ligaments and Achilles tendons of Bmal1-/- mice. Ligament-derived Bmal1-/- fibroblasts showed an osteoblast-like phenotype, upregulated osteogenic and chondrogenic markers, and activated TGFβ/BMP signaling, which was enhanced by TGFβ1 stimulation. Furthermore, Bmal1-/- mouse embryonic fibroblasts had a stronger potential for osteogenic differentiation with activation of TGFβ/BMP signaling.
Conclusions
These findings demonstrated that Bmal1 negatively regulates endochondral ossification in heterotopic ossification of tendons and ligaments with aging via TGFβ/BMP signaling, thereby identifying a new regulatory mechanism in age-related heterotopic ossification of tendons and ligaments.






Similar content being viewed by others
References
Q Zhang D Zhou H Wang J Tan 2020 Heterotopic ossification of tendon and ligament J Cell Mol Med 24:5428–5437. https://doi.org/10.1111/jcmm.15240
R Xu J Hu X Zhou Y Yang 2018 Heterotopic ossification: Mechanistic insights and clinical challenges Bone 109:134–142. https://doi.org/10.1016/j.bone.2017.08.025
S Shahab-Osterloh F Witte A Hoffmann A Winkel S Laggies B Neumann V Seiffart W Lindenmaier AD Gruber J Ringe T Häupl F Thorey E Willbold P Corbeau G Gross 2010 Mesenchymal stem cell-dependent formation of heterotopic tendon-bone insertions (osteotendinous junctions) Stem Cells 28:1590–1601. https://doi.org/10.1002/stem.487
PJ Richards JC Braid MR Carmont N Maffulli 2008 Achilles tendon ossification: pathology, imaging and aetiology Disabil Rehabil 30:1651–1665. https://doi.org/10.1080/09638280701785866
K Saetia D Cho S Lee DH Kim SD Kim 2011 Ossification of the posterior longitudinal ligament: a review Neurosurg Focus. https://doi.org/10.3171/2010.11.FOCUS10276
EJO O'Brien CB Frank NG Shrive B Hallgrímsson DA Hart 2012 Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma Int J Exp Pathol 93:319–331. https://doi.org/10.1111/j.1365-2613.2012.00829.x
K Zhang S Asai MW Hast M Liu Y Usami M Iwamoto LJ Soslowsky M Enomoto-Iwamoto 2016 Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model Matrix Biol 52–54:315–324. https://doi.org/10.1016/j.matbio.2016.01.015
T Iwasawa K Iwasaki T Sawada A Okada K Ueyama S Motomura S Harata I Inoue S Toh KI Furukawa 2006 Pathophysiological role of endothelin in ectopic ossification of human spinal ligaments induced by mechanical stress Calcif Tissue Int 79:422–430. https://doi.org/10.1007/s00223-006-0147-7
S Matsunaga M Kukita K Hayashi R Shinkura C Koriyama T Sakou S Komiya 2002 Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament J Neurosurg 96:168–172. https://doi.org/10.3171/spi.2002.96.2.0168
BS Boody M Lendner AR Vaccaro 2019 Ossification of the posterior longitudinal ligament in the cervical spine: a review Int Orthop 43:797–805. https://doi.org/10.1007/s00264-018-4106-5
P Sharma N Maffulli 2005 Tendon injury and tendinopathy: healing and repair J Bone Joint Surg Am 87:187–202. https://doi.org/10.2106/JBJS.D.01850
OG Davies Y Liu DJ Player NRW Martin LM Grover MP Lewis 2017 Defining the balance between regeneration and pathological ossification in skeletal muscle following traumatic injury Front Physiol 8:194. https://doi.org/10.3389/fphys.2017.00194
X Wang F Li L Xie J Crane G Zhen Y Mishina R Deng B Gao H Chen S Liu P Yang M Gao M Tu Y Wang M Wan C Fan X Cao 2018 Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice Nat Commun 9:551. https://doi.org/10.1038/s41467-018-02988-5
PP Yee Lui YM Wong YF Rui YW Lee LS Chan KM Chan 2011 Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy J Orthop Res 29:816–821. https://doi.org/10.1002/jor.21313
X Qu Z Chen D Fan S Xiang C Sun Y Zeng W Li Z Guo Q Qi W Zhong Y Jiang 2017 Two novel BMP-2 variants identified in patients with thoracic ossification of the ligamentum flavum Eur J Hum Genet 25:565–571. https://doi.org/10.1038/ejhg.2017.2
Yan L, Gao R, Liu Y, He B, Lv S, Hao D (2017) The pathogenesis of ossification of the posterior longitudinal ligament. Aging Dis 8:570–82. https://doi.org/10.14336/AD.2017.0201
NA Agabalyan DJR Evans RL Stanley 2013 Investigating tendon mineralisation in the avian hindlimb: a model for tendon ageing, injury and disease J Anat 223:262–277. https://doi.org/10.1111/joa.12078
G Dai Y Li J Liu C Zhang M Chen P Lu Y Rui 2020 Higher BMP expression in tendon stem/progenitor cells contributes to the increased heterotopic ossification in Achilles tendon with aging Front Cell Dev Biol 8:570605. https://doi.org/10.3389/fcell.2020.570605
G Kobashi M Washio K Okamoto S Sasaki T Yokoyama Y Miyake N Sakamoto K Ohta Y Inaba H Tanaka 2004 High body mass index after age 20 and diabetes mellitus are independent risk factors for ossification of the posterior longitudinal ligament of the spine in Japanese subjects: a case-control study in multiple hospitals Spine 29:1006–1010. https://doi.org/10.1097/00007632-200405010-00011
T Yamauchi E Taketomi S Matsunaga T Sakou 1999 Bone mineral density in patients with ossification of the posterior longitudinal ligament in the cervical spine J Bone Miner Metab 17:296–300. https://doi.org/10.1007/s007740050098
K Mori S Imai T Kasahara K Nishizawa T Mimura Y Matsusue 2014 Prevalence, distribution, and morphology of thoracic ossification of the posterior longitudinal ligament in Japanese: results of CT-based cross-sectional study Spine 39:394–399. https://doi.org/10.1097/BRS.0000000000000153
JR Peterson ON Eboda RC Brownley KE Cilwa LE Pratt S Rosa De La S Agarwal SR Buchman PS Cederna MD Morris SC Wang B Levi 2015 Effects of aging on osteogenic response and heterotopic ossification following burn injury in mice Stem Cells Dev 24:205–213. https://doi.org/10.1089/scd.2014.0291
RV Kondratov AA Kondratova VY Gorbacheva OV Vykhovanets MP Antoch 2006 Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock Genes Dev 20:1868–1873. https://doi.org/10.1101/gad.1432206
MK Bunger LD Wilsbacher SM Moran C Clendenin LA Radcliffe JB Hogenesch MC Simon JS Takahashi CA Bradfield 2000 Mop3 is an essential component of the master circadian pacemaker in mammals Cell 103:1009–1017. https://doi.org/10.1016/s0092-8674(00)00205-1
K-F Storch O Lipan I Leykin N Viswanathan FC Davis WH Wong CJ Weitz 2002 Extensive and divergent circadian gene expression in liver and heart Nature 417:78–83. https://doi.org/10.1038/nature744
MK Bunger JA Walisser R Sullivan PA Manley SM Moran VL Kalscheur RJ Colman CA Bradfield 2005 Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus Genesis 41:122–132. https://doi.org/10.1002/gene.20102
EL McDearmon KN Patel CH Ko JA Walisser AC Schook JL Chong LD Wilsbacher EJ Song H-K Hong CA Bradfield JS Takahashi 2006 Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice Science 314:1304–1308. https://doi.org/10.1126/science.1132430
Y Chen X Wang H Yang J Miao X Liu D Chen 2014 Upregulated expression of PERK in spinal ligament fibroblasts from the patients with ossification of the posterior longitudinal ligament Eur Spine J 23:447–454. https://doi.org/10.1007/s00586-013-3053-5
J Huang S-X Yuan D-X Wang Q-X Wu X Wang C-J Pi X Zou L Chen L-J Ying K Wu J-Q Yang W-J Sun Z-L Deng B-C He 2014 The role of COX-2 in mediating the effect of PTEN on BMP9 induced osteogenic differentiation in mouse embryonic fibroblasts Biomaterials 35:9649–9659. https://doi.org/10.1016/j.biomaterials.2014.08.016
MF Ahmed AK El-Sayed H Chen R Zhao K Jin Q Zuo Y Zhang B Li 2019 Direct conversion of mouse embryonic fibroblast to osteoblast cells using hLMP-3 with Yamanaka factors Int J Biochem Cell Biol 106:84–95. https://doi.org/10.1016/j.biocel.2018.11.008
H Suzuki Y Ito M Shinohara S Yamashita S Ichinose A Kishida T Oyaizu T Kayama R Nakamichi N Koda K Yagishita MK Lotz A Okawa H Asahara 2016 Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis Proc Natl Acad Sci USA 113:7840–7845. https://doi.org/10.1073/pnas.1522054113
EA Schroder BD Harfmann X Zhang R Srikuea JH England BA Hodge Y Wen LA Riley Q Yu A Christie JD Smith T Seward EM Wolf Horrell J Mula CA Peterson TA Butterfield KA Esser 2015 Intrinsic muscle clock is necessary for musculoskeletal health J Physiol 593:5387–5404. https://doi.org/10.1113/JP271436
M Dudek N Gossan N Yang H-J Im JPD Ruckshanthi H Yoshitane X Li D Jin P Wang M Boudiffa I Bellantuono Y Fukada RP Boot-Handford Q-J Meng 2016 The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity J Clin Invest 126:365–376. https://doi.org/10.1172/JCI82755
S Yu Q Tang M Xie X Zhou Y Long Y Xie F Guo L Chen 2019 Circadian BMAL1 regulates mandibular condyle development by hedgehog pathway Cell Prolif. https://doi.org/10.1111/cpr.12727
D Nam B Guo S Chatterjee M-H Chen D Nelson VK Yechoor K Ma 2015 The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways J Cell Sci 128:1835–1847. https://doi.org/10.1242/jcs.167643
H Tasaki L Zhao K Isayama H Chen N Yamauchi Y Shigeyoshi S Hashimoto M-a Hattori 2015 Inhibitory role of REV-ERBα in the expression of bone morphogenetic protein gene family in rat uterus endometrium stromal cells Am J Physiol Cell Physiol 308:528–538. https://doi.org/10.1152/ajpcell.00220.2014
M Dudek N Gossan N Yang H-J Im JPD Ruckshanthi H Yoshitane X Li D Jin P Wang M Boudiffa I Bellantuono Y Fukada RP Boot-Handford Q-J Meng 2015 The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity J Clin Invest 126:365–376. https://doi.org/10.1172/jci82755
Z Qian Y Zhang X Kang H Li Y Zhang X Jin X Gao M Xu Z Ma L Zhao Z Zhang H Sun S Wu 2020 Postnatal conditional deletion of Bmal1 in osteoblasts enhances trabecular bone formation via increased BMP2 signals J Bone Miner Res 35:1481–1493. https://doi.org/10.1002/jbmr.4017
Z Fang T Zhu WL Shen QM Tang JL Chen Z Yin JF Ji BC Heng HW Ouyang X Chen 2014 Transplantation of fetal instead of adult fibroblasts reduces the probability of ectopic ossification during tendon repair Tissue Eng Part A 20:1815–1826. https://doi.org/10.1089/ten.TEA.2013.0296
Y Harada K-I Furukawa T Asari S Chin A Ono T Tanaka H Mizukami M Murakami S Yagihashi S Motomura Y Ishibashi 2014 Osteogenic lineage commitment of mesenchymal stem cells from patients with ossification of the posterior longitudinal ligament Biochem Biophys Res Commun 443:1014–1020. https://doi.org/10.1016/j.bbrc.2013.12.080
RS Archer JI Bayley CW Archer SY Ali 1993 Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons J Anat 182:1–11.
Y Li G Dai L Shi Y Lin M Chen G Li Y Rui 2019 The potential roles of tendon stem/progenitor cells in tendon aging Curr Stem Cell Res Ther 14:34–42. https://doi.org/10.2174/1574888X13666181017112233
KA Dyar S Ciciliot LE Wright RS Biensø GM Tagliazucchi VR Patel M Forcato MI Paz A Gudiksen F Solagna M Albiero 2014 Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock Mol Metab 3:29–41. https://doi.org/10.1016/j.molmet.2013.10.005
S Ray UK Valekunja A Stangherlin SA Howell AP Snijders G Damodaran AB Reddy 2020 Circadian rhythms in the absence of the clock gene Science 367:800–806. https://doi.org/10.1126/science.aaw7365
R Akagi Y Akatsu KM Fisch O Alvarez-Garcia T Teramura Y Muramatsu M Saito T Sasho AI Su MK Lotz 2017 Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-β signaling in chondrocytes Osteoarthr Cartil 25:943–951. https://doi.org/10.1016/j.joca.2016.11.007
N Gossan L Zeef J Hensman A Hughes JF Bateman L Rowley CB Little HD Piggins M Rattray RP Boot-Handford QJ Meng 2013 The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis Arthr Rheum 65:2334–2345. https://doi.org/10.1002/art.38035
L Fu MS Patel A Bradley EF Wagner G Karsenty 2005 The molecular clock mediates leptin-regulated bone formation Cell 122:803–815. https://doi.org/10.1016/j.cell.2005.06.028
WD Chen JK Yeh MT Peng SS Shie SL Lin CH Yang TH Chen KC Hung CC Wang IC Hsieh MS Wen CY Wang 2015 Circadian CLOCK mediates activation of transforming growth factor-β signaling and renal fibrosis through cyclooxygenase 2 Am J Pathol 185:3152–3163. https://doi.org/10.1016/j.ajpath.2015.08.003
G Chen Q Tang S Yu Y Xie J Sun S Li L Chen 2020 The biological function of BMAL1 in skeleton development and disorders Life Sci 253:117636. https://doi.org/10.1016/j.lfs.2020.117636
Acknowledgements
We thank the members of the Zhu lab for their helpful discussion.
Funding
This work was supported by the Research Start-up Fund of the Seventh Affiliated Hospital, Sun Yat-sen University (ZSQYBRJH0003).
Author information
Authors and Affiliations
Contributions
CMZ, YL, and SYL conceived and designed the project. QL, YSL, and LY designed and performed experiments, analyzed data, wrote, and edited the manuscript. QQZ, WLX, and YW performed experiments and analyzed data. LPY and QJL designed experiments and critically revised the manuscript. All the authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethics Approval
All animal experiments were approved by the Sun Yat-sen University Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
774_2021_1271_MOESM1_ESM.tif
Supplementary file1 (TIF 459 KB) Fig. S1 Reduction of Bmal1 expressions in mouse PLLs and Achilles tendons with aging. a The mRNA levels of Bmal1 in PLLs from 6- and 32-weeks WT mice. PLLs, posterior longitudinal ligaments. b The gene expressions of Bmal1 in Achilles tendons from WT mice at 6 weeks and 32 weeks. Data are presented as the mean ± SD (n = 6 mice/group). **P < 0.01, ***P < 0.001
774_2021_1271_MOESM2_ESM.tif
Supplementary file2 (TIF 174 KB) Fig. S2 Quantitative analysis of HO volume in the PLLs (a) and Achilles tendons (b) from Bmal1 KO and WT mice at 6, 12, 18, and 32 weeks of age. Data are presented as the mean ± SD (n = 6 mice/group). *P < 0.05
774_2021_1271_MOESM3_ESM.tif
Supplementary file3 (TIF 431 KB) Fig. S3 The cell surface antigens CD29 and CD90 on mouse PLL- derived cells. a Flow cytometric analysis of cell surface antigens CD29 and CD90 on mouse PLL- derived cells. b Percentages of CD29+CD90- cells between Bmal1 KO and WT mice. Data are presented as the mean ± SD (n = 6 mice/group); ns, not significant
About this article
Cite this article
Liang, Q., Lu, Y., Yu, L. et al. Disruption of the mouse Bmal1 locus promotes heterotopic ossification with aging via TGF-beta/BMP signaling. J Bone Miner Metab 40, 40–55 (2022). https://doi.org/10.1007/s00774-021-01271-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01271-w