Abstract
Introduction
The occurrence of early adverse events and the factors associated with these events in zoledronic acid-treated Japanese patients with osteoporosis were investigated.
Materials and methods
All patients treated with zoledronic acid for the first time for primary osteoporosis were analyzed. Based on the history of bisphosphonate (BP) administration, the patients were divided into three groups: BP-switch, BP-washout, and naïve groups. The BP-washout and naive groups were combined into a non-BP group.
Results
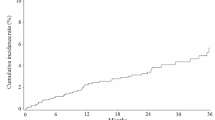
A total of 184 patients with a mean age of 77.4 years were included. Acute phase reactions (APRs) occurred in 32 patients (17.4%). The significant risk factors were hospitalization (vs. outpatients), BP-switch (vs. non-BP), and age > 80 years (vs. ≤ 69 years), and the odds ratios were 5.63, 0.12, and 0.23, respectively. The serum calcium levels were significantly reduced in the non-BP group, regardless of the co-administration of active vitamin D3. However, the patients who were co-administered active vitamin D3 had significantly higher values than those who were not. In the BP-switch group, no significant reduction in serum calcium levels was observed; however, the reductions tended to be smaller in the patients who were co-administered active vitamin D3.
Conclusion
Occurrence of APRs might be lesser in clinical practice than in phase 3 clinical trials. Although serum calcium levels decreased in many cases, the decrease could be suppressed by the co-administration of active vitamin D3.

Similar content being viewed by others
References
Nakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, Sone T, Taguchi A, Tanaka S, Ohashi M, Ota Y, Shiraki M (2017) Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos Int 28:389–398
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254
Zhou J, Ma X, Wang T, Zhai S (2016) Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int 27:3289–3300
Eastell R, Black DM, Boonen S (2009) Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 94:3215–3225
Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J et al (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367:1714–1723
Reid IR, Gamble GD, Mesenbrink P, Lakatps P, Black DM (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 95:4380–4387
Hwang JS, Chin LS, Chen JF, Yang TS, Chen PQ, Tsai KS, Leung PC (2011) The effects of intravenous zoledronic acid in Chinese women with postmenopausal osteoporosis. J Bone Miner Metab 29:328–333
Popp AW, Senn R, Curkovic I, Senn C, Buffat H, Popp PF, Lippuner K (2017) Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int 28:1995–2002
Okimoto N, Sakai A, Yoshioka T, Kobayashi T, Asano K, Akahoshi S, Ishikura T, Fukuhara S, Fuse Y, Mizuno T, Katae Y, Matsumoto H, Ogawa T, Nishida S, Ikeda S, Menuki K, Saito J, Okazaki Y, Mizuno N, Fujiwara S (2020) Efficacy of non-steroidal anti-inflammatory drugs on zoledronic acid-induced acute-phase reactions: randomized, open-label, Japanese OZ study. J Bone Miner Metab 38:230–239
Thompson K, Rogers MJ (2004) Statins prevent bisphosphonate-induced γ, δ-T-cell proliferation and activation in vitro. J Bone Miner Res 19:278–288
Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T, Morii H, Tomita A, Yamamoto K, Nagata Y, Nakashima M, Orimo H (1999) A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int 10:183–192
Suzuki T, Kwon J, Kim H, Shimada H, Yoshida Y, Iwasa H, Yoshida H (2008) Low serum 25-hydroxyvitamin D levels associated with falls among Japanese community-dwelling elderly. J Bone Miner Res 23:1309–1317
Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 367:40–49
Acknowledgements
This research was supported by the Asahi Kasei Pharma Corporation.
Author information
Authors and Affiliations
Contributions
JT and KI designed the study, performed formal analysis, and wrote the original draft. JT, KI, OY, TD, and AS collected the data. JT, KI, MY, TT, and TA analyzed the data. JT, KI, and TY edited the final manuscript. All authors read and approved drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JT received lecture and advisor fees from Asahi Kasei Pharma Corporation. The other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takada, J., Iba, K., Yamamoto, O. et al. Early adverse events after the first administration of zoledronic acid in Japanese patients with osteoporosis. J Bone Miner Metab 39, 903–910 (2021). https://doi.org/10.1007/s00774-021-01231-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-021-01231-4