Abstract
Introduction
Only a few large-scale studies have examined the care gap in Japan. The aim of this study was to investigate the persistence of and adherence to osteoporosis pharmacotherapy in Japan.
Materials and Methods
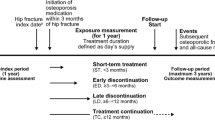
The rates of continuation (persistence) of and adherence to osteoporosis pharmacotherapy were investigated using medical insurance data, issued from July 2013 to December 2018, from the medical care system for elderly individuals in Hokkaido, Japan.
Results
The study included 7918 male and 52,585 female patients. Persistence rates were 62.1% in the first year and 45.3% in the second year. There were 33,096 patients who discontinued medication; 8296 patients resumed medication during the observation period of 730 days. The median time to the discontinuation of medication for all the patients was 702 days. The 2-year medication possession ratio (MPR) was 63.8%; 30,989 patients (51.2%) had an MPR ≥ 80% and 20,788 (34.4%) had an MPR < 50%. Both the persistence and adherence were better in females than in males and worsened with increasing age. Comparisons of fracture history showed that persistence and MPR were higher in the no hip or vertebral fracture group, followed by hip fracture, vertebral fracture, and hip and vertebral fracture groups. Meanwhile, more patients in the hip fracture group had an MPR ≥ 80%.
Conclusion
Persistence of and adherence to osteoporotic pharmacotherapy are not very high in Japan. To bridge the care gap following osteoporosis pharmacotherapy, improvements are required for males, the elderly, and those with a history of vertebral fracture.


Similar content being viewed by others
References
Melton LJ 3rd, Achenbach SJ, Atkinson EJ, Therneau TM, Amin S (2013) Long-term mortality following fractures at different skeletal sites: a population-based cohort study. Osteoporos Int 24:1689–1696
Svedbom A, Borgstöm F, Hernlund E, Ström O, Alekna V, Bianchi ML, Clark P, Curiel MD, Dimai HP, Jürisson M, Kallikorm R (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures—results from the ICUROS. Osteoporos Int 29:557–566
Orimo H, Yaegashi Y, Hosoi T, Fukushima Y, Onoda T, Hashimoto T, Sakata K (2016) Hip fracture incidence in Japan: estimates of new patients in 2012 and 25-year trends. Osteoporos Int 27:1777–1784
Cheung CL, Ang SB, Chadha M, Chow ES, Chung YS, Hew FL, Jaisamrarn U, Ng H, Takeuchi Y, Wu CH, Xia W, Yu J, Fujiwara S (2018) An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia 4:16–21
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fract Interv Trial Res Group Lancet 348:1535–1541
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY, Hip Intervention Program Study Group (2001) Effect of risedronate on the risk of hip fracture in elderly women Hip Intervention Program Study Group. N Engl J Med 344:333–340
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, FREEDOM Trial (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Chen YC, Lin WC (2017) Poor 1st-year adherence to anti-osteoporotic therapy increases the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures. Patient Prefer Adherence 11:839–843
Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R (2011) Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int 22:2565–2573
Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY (2010) Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int 86:202–210
Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E (2011) A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 14:571–581
Imaz I, Zegarra P, González-Enríquez J, Rubio B, Alcazar R, Amate JM (2010) Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 21:1943–1951
Fatoye F, Smith P, Gebrye T, Yeowell G (2019) Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9:e027049
Yeam CT, Chia S, Tan HCC, Kwan YH, Fong W, Seng JJB (2018) A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int 29:2623–2637
Fardellone P, Lello S, Cano A, de Sá ME, Watanabe de Oliveira R, Julian GS, Tang B (2019) Real-world adherence and persistence with bisphosphonate therapy in postmenopausal women: a systematic review. Clin Ther 41:1576–1588
van Boven JF, de Boer PT, Postma MJ, Vegter S (2013) Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 31:562–570
Keshishian A, Boytsov N, Burge R, Krohn K, Lombard L, Zhang X, Xie L, Baser O (2017) Examining the effect of medication adherence on risk of subsequent fracture among women with a fragility fracture in the U.S. medicare population. J Manag Care Spec Pharm 23:1178–1190
Sato M, Tsujimoto M, Kajimoto K, Uetake H, Shimoda H, Fujiwara S (2018) Effect of a patient-support program on once-daily teriparatide adherence and persistence in the Japan Fracture Observational Study (JFOS). Arch Osteoporos 13:74
Kishimoto H, Maehara M (2015) Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos 10:231
Sakai A, Ikeda S, Okimoto N, Matsumoto H, Teshima K, Okazaki Y, Fukuda F, Arita S, Tsurukami H, Nagashima M, Yoshioka T (2014) Clinical efficacy and treatment persistence of monthly minodronate for osteoporotic patients unsatisfied with, and shifted from, daily or weekly bisphosphonates: the BP-MUSASHI study. Osteoporos Int 25:2245–2253
Ministry of Health, Labour, and Welfare. The popularization and state of electronic receipt claims. https://www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000099002.pdf. (in Japanese). Accessed 11 Feb 2020
Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, Sherrington C, Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group (2016) A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 16:158
Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S (2012) Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 19:33–40
Desai RJ, Mahesri M, Abdia Y, Barberio J, Tong A, Zhang D, Mavros P, Kim SC, Franklin JM (2018) Association of osteoporosis medication use after hip fracture with prevention of subsequent nonvertebral fractures: an instrumental variable analysis. JAMA Netw Open 1:e180826
Cheung MY, Ho AW, Wong SH (2018) Post-fracture care gap: a retrospective population-based analysis of Hong Kong from 2009 to 2012. Hong Kong Med J 24:579–583
Nakatoh S, Fujimori K, Tamaki J, Okimoto N, Ogawa S, Iki M (2020) Insufficient increase in bone mineral density testing rates and pharmacotherapy after hip fracture in Japan. J Bone Miner Metab 38(4):589–596
Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R (2017) Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 12:22
Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, Prieto-Alhambra D (2017) One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 28:2997–3004
Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD (2007) Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging 24:37–55
Roerholt C, Eiken P, Abrahamsen B (2009) Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 20:299–307
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Iversen MD, Vora RR, Servi A, Solomon DH (2011) Factors affecting adherence to osteoporosis medications: a focus group approach examining viewpoints of patients and providers. J Geriatr Phys Ther 34:72–81
Neele SJ, Evertz R, De Valk-De RG, Roos JC, Netelenbos JC (2002) Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone 30:599–603
Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20:1507–1513
Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19:365–372
Nakatoh S (2017) Bone turnover rate and bone formation/resorption balance during the early stage after switching from a bone resorption inhibitor to denosumab are predictive factors of bone mineral density change. Osteoporos Sarcopenia 3:45–52
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res 32:1291–1296
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28:1723–1732
Japan Osteoporosis Society (2015) Guidelines on the prevention and treatment of osteoporosis 2015. The committee for development of the guidelines on the prevention and treatment of osteoporosis. (in Japanese). http://www.josteo.com/ja/guideline/doc/15_1.pdf. Accessed 11 Feb 2020
Forbes CA, Deshpande S, Sorio-Vilela F, Kutikova L, Duffy S, Gouni-Berthold I, Hagström E (2018) A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin 34:1613–1625
Cabinet office. The state of aging. https://www8.cao.go.jp/kourei/whitepaper/w-2017/html/gaiyou/s1_1.html. (in Japanese). Accessed 11 Feb 2020
Geospatial Information Authority of Japan. Area according to administrative divisions. https://www.gsi.go.jp/KOKUJYOHO/MENCHO/201910/area_todofuken.pdf. (in Japanese). Accessed 11 Feb 2020
Tamaki J, Fujimori K, Ikehara S, Kamiya K, Nakatoh S, Okimoto N, Ogawa S, Ishii S, Iki M, Working Group of Japan Osteoporosis Foundation (2019) Estimates of hip fracture incidence in Japan using the National Health Insurance Claim Database in 2012–2015. Osteoporos Int 30:975–983
Acknowledgements
This study did not receive any funding from agencies in the public, commercial, or not-for profit sectors.
Author information
Authors and Affiliations
Contributions
SN: conception and design, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content, and final approval. KF had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. JT, NO, MI, JO: conception and design, revising the article critically for important intellectual content, and final approval.
Corresponding author
Ethics declarations
Conflict of interest
S. Nakatoh has received lecture fees from Asahi-Kasei Pharmaceutical Co., Ltd.; Pfizer Japan Inc.; Amgen Astellas BioPharma K.K.; Astellas Pharma Inc.; Daiichi-Sankyo Co. Ltd.; and Eli Lilly Japan K.K. N. Okimoto has received consulting fees from Asahi-Kasei Pharmaceutical Co., Ltd and Teijin Pharma Ltd. N. Okimoto has received payments for lectures, including speakers’ bureau fees, from Asahi-Kasei Pharmaceutical Co., Ltd.; Amgen Astellas BioPharma K.K.; Astellas Pharma Inc.; Chugai Pharmaceutical Co.; Daiichi-Sankyo Co. Ltd.; Eisai Co., Ltd.; Eli Lilly Japan K.K.; Mitsubishi-Tanabe Pharma Corp.; Ono Pharmaceutical Co.; Pfizer Japan Inc.; and Teijin Pharma Ltd. The other authors have no conflicts of interest.
Ethical approval
This study was conducted according to the principles of the Declaration of Helsinki and approved by the institutional review board of the Asahi General Hospital (IRB No. 19-02) and the ethics committee of Tohoku University as “Examination of drug treatment compliance in osteoporosis using an electronic claim database” (2018-1-868). This study was a part of the “medical/nursing care information database development project,” in which the National Health Insurance Federation was instructed by the Hokkaido Government to construct the database at Tohoku University. The claim data, which were collected by KF, were provided by the governor with the approval by all municipal governments after anonymization in the National Health Insurance Federation.
Informed consent
The data in this study were completely anonymous; thus, informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakatoh, S., Fujimori, K., Tamaki, J. et al. Insufficient persistence of and adherence to osteoporosis pharmacotherapy in Japan. J Bone Miner Metab 39, 501–509 (2021). https://doi.org/10.1007/s00774-020-01188-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01188-w