Abstract
Introduction
Etelcalcetide (Parsabiv®, AMG 416/ONO-5163) is a novel allosteric modulator for the calcium-sensing receptor approved for hemodialysis patients with secondary hyperparathyroidism of uremia. Etelcalcetide reduced parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism of uremia in clinical studies. However, its direct effect on parathyroid hormone secretion in human parathyroid cells remains unknown. This study aimed to determine if etelcalcetide suppresses parathyroid hormone secretion by human parathyroid cells in vitro.
Materials and methods
We prepared primary cell cultures from human parathyroid tissue and determined calcium-sensing receptor expression levels by immunohistochemistry. Pathyroid tumors were removed from fourteen patients with primary hyperparathyrodism. Parathyroid tissue was dispersed with collagenase, resuspended in culture medium, incubated for 2 h with etelcalcetide and Ca2+, and the medium was then collected. Final etelcalcetide concentrations in the medium were 0.005–50 µmol/L. Levels of human parathyroid hormone in the medium were determined by enzyme-linked immunosorbent assay.
Results
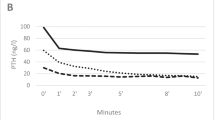
In eight of the fourteen parathyroid cell cultures, extracellular Ca2+ reduced parathyroid hormone levels. In four of the eight parathyroid cell cultures which responded extracellular Ca2+, etelcalcetide reduced hormone secretion with the 50% effective concentrations of 0.57, 20.8, 0.42, and 0.57 µmol/L. Expression levels of the calcium-sensing receptor were significantly lower in primary hyperparathyroidism patient-derived parathyroid tissues compared with controls.
Conclusion
This is the first report that etelcalcetide directly reduced parathyroid hormone secretion from the primary cultured human parathyroid cells from patients with primary hyperparathyroidism. To verify this conclusion, further studies are needed using secondary hyperparathyroidism patient-derived parathyroid cells.




Similar content being viewed by others
References
Cunningham J, Locatelli F, Rodriguez M (2011) Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6:913–921
Joy MS, Karagiannis PC, Peyerl FW (2007) Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm 13:397–411
Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D (2004) Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308:627–635
Walter S, Baruch A, Dong J, Tomlinson JE, Alexander ST, Janes J, Hunter T, Yin Q, Maclean D, Bell G, Mendel DB, Johnson RM, Karim F (2013) Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther 346:229–240
Blair HA (2013) Etelcalcetide: First global approval. Drugs 76:1787–1792
Inoue A, Harada K (2017) The discovery, research and development of etelcalcetide hydrochloride, the world 1st intravenous calcimimetics (in Japanese). Clin Calcium 27:537–545
Alexander ST, Hunter T, Walter S, Dong J, Maclean D, Baruch A, Subramanian R, Tomlinson JE (2015) Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol 88:853–865
Harada K, Inoue A, Yamauchi A, Fujii A (2017) The pharmacological profile and the clinical efficacy of the world’s 1st intravenous calcimimetics; etelcalcetide hydrochloride (Parsabiv®) (in Japanese). Nippon Yakurigaku Zasshi (Folia Pharmacol Jpn) 150:98–113
Harada K, Fujioka A, Konno M, Inoue A, Yamada H, Hirota Y (2019) Pharmacology of Parsabiv® (etelcalcetide, ONO-5163/AMG 416), a novel allosteric modulator of the calcium-sensing receptor, for secondary hyperparathyroidism in hemodialysis patients. Eur J Pharmacol 842:139–145
Block GA, Bushinsky DA, Cheng S, Cunningham J, Dehmel B, Drueke TB, Ketteler M, Kewalramani R, Martin KJ, Moe SM, Patel UD, Silver J, Sun Y, Wang H, Chertow GM (2017) Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 317:156–164
Fukagawa M, Yokoyama K, Shigematsu T, Akiba T, Fujii A, Kuramoto T, Odani M, Akizawa T, ONO-5163 Study Group (2017) A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 32:1723–1730
Bilezikian JP, Potts JT Jr, Gel-H F, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA Jr (2002) Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361
Kawata T, Imanishi Y, Kobayashi K, Onoda N, Okuno S, Takemoto Y, Komo T, Tahara H, Wada M, Nagano N, Ishimura E, Miki T, Ishikawa T, Inaba M, Nishizawa Y (2006) Direct in vitro evidence of the suppressive effect of cinacalcet HCl on parathyroid hormone secretion in human parathyroid cells with pathologically reduced calcium-sensing receptor levels. J Bone Miner Metab 24:300–306
Ono Pharmaceutical Co., Ltd., Etelcalcetide application dossier CTD 2.6.2.6. (in Japanese). https://www.pmda.go.jp/drugs/2016/P20161220003/index.html. 2016. Accessed 21 Apr 2020
Brown AJ, Zhong M, Ritter C, Brown EM, Slatoptlsky E (1995) Loss of calcium responsiveness in cultured bovine parathyroid cells is associated with decreased calcium receptor expression. Biochem Biophys Res Commun 212:861–867
Tominaga Y, Kakuta T, Yasunaga C, Nakamura M, Kadokura Y, Tahara H (2016) Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the Parathyroid Surgeons’ Society of Japan. Ther Apher Dial 20:6–11
Imanishi Y, Inaba M, Kawata T, Nishizawa Y (2009) Animal models of hyperfunctioning parathyroid diseases for drug development. Expert Opin Drug Discov 4:727–740
Cunningham J, Block GA, Chertow GM, Cooper K, Evenepoel P, Iles J, Sun Y, Ureña-Torres P, Bushinsky DA (2019) Etelcalcetide is effective at all levels of severity of secondary hyperparathyroidism in hemodialysis patients. Kidney Int Rep 4:987–994
Acknowledgements
We express our sincere gratitude to William W. Stark Jr., Audrey Hou, and Sunfa Cheng, MD, of Amgen Inc. (South San Francisco, CA, USA) for the collaborative research. We also thank our colleague Tamami Arai for preparing the manuscript and Chikara Honda for advise on statistical analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AF designed the study and wrote the main manuscript. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AF, YI, IK, TH, AI, KH, MT, YS, HY, DM and NH. YI, AI, HY, ME and MI reviewed the manuscript critically for intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AF, AI, KH, MT, YS, and HY are employees of Ono Pharmaceutical Co., Ltd.; YI, ME, and MI are on the speaker bureau for Ono Pharmaceutical Co., Ltd.; IK, TH, DM, and NH: none.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fujioka, A., Imanishi, Y., Kobayashi, I. et al. Effect of etelcalcetide on parathyroid hormone secretion by primary hyperparathyroidism patient-derived primary parathyroid cells. J Bone Miner Metab 39, 396–403 (2021). https://doi.org/10.1007/s00774-020-01158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01158-2