Abstract
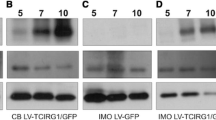
Cell–cell fusion is a critical step in osteoclast development, because only after osteoclasts become multinucleated can they efficiently resorb bone. Although recent studies suggest that dendritic cell-specific transmembrane protein (DC-STAMP) may play a key role in the process of fusion of mouse osteoclasts, little information has been available on the role of DC-STAMP in human osteoclasts. In this study, we screened and identified an in vitro-transcribed short-hairpin RNA targeting human DC-STAMP from four candidates and generated a lentivirus vector. Subsequent experiments indicated that this lentiviral transgenic system could effectively transfer into target human osteoclasts, at more than 80 % gene transfer efficiency at multiplicity of infection of 15, and significantly and specifically inhibited DC-STAMP expression at both mRNA and protein levels. We also found that DC-STAMP inhibition by RNAi consequently suppressed fusion and bone resorption of human osteoclasts. In conclusion, these data indicated the lentivirus-mediated RNAi was capable of efficiently suppressing DC-STAMP expression in primary human osteoclasts and inhibiting osteoclastogenesis, demonstrating an essential role of DC-STAMP in the differentiation of human osteoclasts.




Similar content being viewed by others
References
Yaccoby S, Pearse RN, Johnson CL, Barlogie B, Choi Y, Epstein J (2002) Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol 116:278–290
Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T (2004) Osteoclasts enhance myeloma cell growth and survival via cell–cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104:2484–2491
Iwasaki R, Ninomiya K, Miyamoto K, Suzuki T, Sato Y, Kawana H, Nakagawa T, Suda T, Miyamoto T (2008) Cell fusion in osteoclasts plays a critical role in controlling bone mass and osteoblastic activity. Biochem Biophys Res Commun 377:899–904
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T (2005) DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351
Zeng Z, Chen J (2009) Cell–cell fusion: human multinucleated osteoclasts. Cent Eur J Biol 4:543–548
Chiu YH, Mensah KA, Schwarz EM, Ju Y, Takahata M, Feng C, McMahon LA, Hicks DG, Panepento B, Keng PC, Ritchlin CT (2011) Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP). J Bone Miner Res 43:685–689
Laitala-Leinonen T (2005) Unsatisfactory gene transfer into bone-resorbing osteoclasts with liposomal transfection systems. J Negat Results Biomed 4:5
Fukuda A, Hikita A, Wakeyama H, Akiyama T, Oda H, Nakamura K, Tanaka S (2005) Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res 20:2245–2253
Kobayashi Y, Take I, Yamashita T, Mizoguchi T, Ninomiya T, Hattori T, Kurihara S, Ozawa H, Udagawa N, Takahashi N (2005) Prostaglandin E2 receptors EP2 and EP4 are down-regulated during differentiation of mouse osteoclasts from their precursors. J Biol Chem 280:24035–24042
Zeng Z, Chen J (2012) Construction and identification of eukaryotic expression vector expressing human DC-STAMP. J Fujian Med Univ 46:31–35
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature (Lond) 423:337–342
Kanamaru F, Iwai H, Ikeda T, Nakajima A, Ishikawa I, Azuma M (2004) Expression of membrane-bound and soluble receptor activator of NF-kappaB ligand (RANKL) in human T cells. Immunol Lett 94:239–246
Itonaga I, Sabokbar A, Neale SD, Athanasou NA (1999) 1,25-Dihydroxyvitamin D(3) and prostaglandin E(2) act directly on circulating human osteoclast precursors. Biochem Biophys Res Commun 264:590–595
Kotake S, Yago T, Kawamoto M, Nanke Y (2012) Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial ‘human osteoclastology’. J Bone Miner Metab 30:125–135
Kotake S, Nanke Y, Yago T, Kawamoto M, Yamanaka H (2009) Human osteoclastogenic T cells and human osteoclastology. Arthritis Rheum 60:3158–3163
Hartgers FC, Vissers JL, Looman MW, van Zoelen C, Huffine C, Figdor CG, Adema GJ (2000) DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur J Immunol 30:3585–3590
Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H (2004) RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 200:941–946
Pushparaj PN, Aarthi JJ, Manikandan J, Kumar SD (2008) siRNA, miRNA, and shRNA: in vivo applications. J Dent Res 87:992–1003
Kasim V, Taira K, Miyagishi M (2006) Screening of siRNA target sequences by using fragmentized DNA. J Gene Med 8:782–791
Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M, Collins FS (2004) Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci USA 101:1892–1897
Chu K, Cornetta KG, Econs MJ (2008) Efficient and stable gene expression into human osteoclasts using an HIV-1-based lentiviral vector. DNA Cell Biol 27:315–320
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30871111 and No. 81172259) and the Natural Science Foundation of Fujian Province of China (No. 2011J05064).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zeng, Z., Zhang, C. & Chen, J. Lentivirus-mediated RNA interference of DC-STAMP expression inhibits the fusion and resorptive activity of human osteoclasts. J Bone Miner Metab 31, 409–416 (2013). https://doi.org/10.1007/s00774-013-0434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0434-0