Abstract
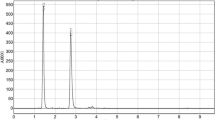
Equol, an exclusive gut bacteria metabolite, is associated with the incidence of low blood pressure, strokes, and hormone-related cancers due to its estrogen-mimicking structure and antioxidant properties. However, all humans cannot produce S-equol upon consumption of soybean and are thus deprived of the health benefits. Existing methods of equol estimation involve complex instrumentation, which is time-consuming and expensive. Therefore, in this study, we develop a simple, cost-effective, accurate, and rapid high-performance thin-layer chromatography (HPTLC) method for equol estimation. Chromatography was performed on precoated silica gel aluminum 60 F254 HPTLC plates, and it produced a compact spot of equol [retardation factor (RF) value of 0.77 ± 0.04] with a mobile phase ethyl acetate–toluene–formic acid in the ratio 5:4:1 (V/V). Ultraviolet detection was performed densitometrically at λmax 200 nm. The method was validated for precision, recovery, robustness, specificity, limit of detection, and limit of quantification, following the International Council for Harmonisation (ICH) guidelines. It was found that S-equol concentration was higher in rats fed with fermented soybean, and the concentration was higher in feces as compared with urine samples. The highest S-equol content in feces and urine was 16.74 µg g−1 and 6.34 µg mL−1, respectively. Quantification of S-equol by HPTLC enables us to segregate the human population into two groups: equol producers/healthy individuals and nonequol producers/nonhealthy individuals. In this era of personalized medicine, such an insight will prove to be of great value and also reduce the drug load on our body.
Graphical abstract






Similar content being viewed by others
References
Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KD (1982) The identification of the weak oestrogen equol [7-hydroxy-3-(4’-hydroxyphenyl)chroman] in human urine. Biochem J 201:353–357. https://doi.org/10.1042/bj2010353
Setchell KDR, Brown NM, Lydeking-Olsen E (2002) The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 132:3577–3584. https://doi.org/10.1093/jn/132.12.3577
Yuan P, Wang H, Liu X (2007) Metabolism of dietary soy isoflavones to equol by human intestinal microflora: implications for health. Mol Nutr Food Res 51:765–781. https://doi.org/10.1002/mnfr.200600262
Magee PJ, Allsopp P, Samaletdin A, Rowland IR (2014) Daidzein, R-(+)equol and S-(-)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur J Nutr 53:345–350. https://doi.org/10.1007/s00394-013-0520-z
Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ (2004) Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod 70:1188–1195. https://doi.org/10.1095/biolreprod.103.023713
Zubik L, Meydani M (2003) Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr 77:1459–1465. https://doi.org/10.1093/ajcn/77.6.1459
Kim M, Kim SI, Han J, Wang XL, Song DG, Kim SU (2009) Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl Environ Microbiol 75:3062–3068. https://doi.org/10.1128/AEM.02058-08
Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE (2005) S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 81:1072–1079. https://doi.org/10.1093/ajcn/81.5.1072
Nagel SC, Vom Saal FS, Welshons WV (1998) The effective free fraction of estradiol and xenoestrogens in human serum measured by whole cell uptake assays: physiology of delivery modifies estrogenic activity. Proc Soc Exp Biol Med 217:300–309. https://doi.org/10.3181/00379727-217-44236
Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA (2004) Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem 12:1559–1567. https://doi.org/10.1016/j.bmc.2003.11.035
Rimbach G, De Pascual-Teresa S, Ewins BA, Matsugo S, Uchida Y, Minihane AM, Turner R, Vafei Adou K, Weinberg PD (2003) Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica 33:913–925. https://doi.org/10.1080/0049825031000150444
Martin ME, Haourigui M, Pelissero C, Benassayag C, Nunez EA (1995) Interactions between phytoestrogens and human sex steroid binding protein. Life Sci 58:429–436. https://doi.org/10.1016/0024-3205(95)02308-9
Garreau B, Vallette G, Adlercreutz H, Wähälä K, Mäkelä T, Benassayag C, Nunez E (1991) Phytoestrogens: new ligands for rat and human α-fetoprotein. Biochim Biophys Acta Mol Cell Res 1094:339–345. https://doi.org/10.1016/0167-4889(91)90095-F
Setchell KDR, Cole SJ (2006) Method of defining equol-producer status and its frequency among vegetarians. J Nutr 136:2188–2193. https://doi.org/10.1093/jn/136.8.2188
Jenks BH, Iwashita S, Nakagawa Y, Ragland K, Lee J, Carson WH, Ueno T, Uchiyama S (2012) A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency. J Womens Health 21:674–682. https://doi.org/10.1089/jwh.2011.3153
Aso T, Uchiyama S, Matsumura Y, Taguchi M, Nozaki M, Takamatsu K, Ishizuka B, Kubota T, Mizunuma H, Ohta H (2012) A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J Womens Health 21:92–100. https://doi.org/10.1089/jwh.2011.2753
Oyama A, Ueno T, Uchiyama S, Aihara T, Miyake A, Kondo S, Matsunaga K (2012) The effects of natural S-equol supplementation on skin aging in postmenopausal women: a pilot randomized placebo-controlled trial. Menopause 19:202–210. https://doi.org/10.1097/gme.0b013e318227427b
Clarkson TB (2002) Soy phytoestrogens and cardiovascular disease. J Nutr 132:566S-569S. https://doi.org/10.1093/jn/132.3.566S
Messina M, Gardner C, Barnes S (2002) Gaining insight into the health effects of soy but a long way still to go: commentary on the fourth International Symposium on the Role of Soy in Preventing and Treating Chronic Disease. J Nutr 132:547S-551S. https://doi.org/10.1093/jn/132.3.547S
Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Mori M (2002) Is daidzein non-metabolizer a high risk for prostate cancer? A case-controlled study of serum soybean isoflavone concentration. Jpn J Clin Oncol 32:296–300. https://doi.org/10.1093/jjco/hyf064
Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, Mori M, Sakauchi F, Washio M, Ito Y, Suzuki K, Wakai K, Tamakoshi A (2004) Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci 95:65–71. https://doi.org/10.1111/j.1349-7006.2004.tb03172.x
Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomark Prev 11:815–821
Wang H, Murphy PA (1994) Isoflavone content in commercial soybean foods. J Agric Food Chem 42:1666–1673. https://doi.org/10.1021/jf00044a016
Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W (2005) Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol 183:45–55. https://doi.org/10.1007/s00203-004-0747-4
Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreyn RF, Mareel MM, De Keukeleire D (2003) Quantitation of soy-derived phytoestrogens in human breast tissue and biological fluids by high-performance liquid chromatography. J Chromatogr B 784:137–144. https://doi.org/10.1016/s1570-0232(02)00789-4
Matthies A, Clavel T, Gütschow M, Engst W, Haller D, Blaut M, Braune A (2008) Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Appl Environ Microbiol 74:4847–4852. https://doi.org/10.1128/AEM.00555-08
Rüfer CE, Glatt H, Kulling SE (2006) Structural elucidation of hydroxylated metabolites of the isoflavan equol by gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry. Drug Metab Dispos 34:51–60. https://doi.org/10.1124/dmd.105.004929
Ko TF, Tsai S, Lin M, Liu D, Learn P, Chiou YY (2010) GC–MS determined distribution of urinary equol producers as affected by age, gender, and repeated ingestions of soymilk. J Food Sci 75:H306–H310. https://doi.org/10.1111/j.1750-3841.2010.01860.x
Moors S, Blaszkewicz M, Bolt HM, Degen GH (2007) Simultaneous determination of daidzein, equol, genistein and bisphenol A in human urine by a fast and simple method using SPE and GC–MS. Mol Nutr Food Res 51:787–798. https://doi.org/10.1002/mnfr.200600289
Grace PB, Mistry NS, Carter MH, Leathem AJ, Teale P (2007) High throughput quantification of phytoestrogens in human urine and serum using liquid chromatography/tandem mass spectrometry (LC–MS/MS). J Chromatogr 853:138–146. https://doi.org/10.1016/j.jchromb.2007.03.011
Kašparovská J, Dadáková K, Lochman J, Hadrová S, Křížová L, Kašparovský T (2017) Changes in equol and major soybean isoflavone contents during processing and storage of yogurts made from control or isoflavone-enriched bovine milk determined using LC–MS (TOF) analysis. Food Chem 222:67–73. https://doi.org/10.1016/j.foodchem.2016.12.010
Manickavasagam L, Gupta S, Mishra S, Maurya R, Chattopadhyay N, Jain GK (2011) LC–MS/MS method for simultaneous analysis of cladrin and equol in rat plasma and its application in pharmacokinetics study of cladrin. Med Chem Res 20:1566–1572. https://doi.org/10.1007/s00044-010-9415-1
Sosvorová L, Lanková P, Bičíková M, Prokudina EA, Al Malarik N, Lapčík O (2011) ELISA for free S-equol in human urine. Czech J Food Sci 29:57–64. https://doi.org/10.17221/130/2010-CJFS
Brouwers E, L’homme R, Al-Maharik N, Lapcík O, Hampl R, Wähälä K, Mikola H, Adlercreutz H (2003) Time-resolved fluoroimmunoassay for equol in plasma and urine. J Steroid Biochem Mol Biol 84:577–587. https://doi.org/10.1016/S0960-0760(03)00071-2
Yokoyama S, Kuzuguchi T (2007) Rapid and convenient detection of urinary equol by thin-layer chromatography. J Nutr Sci Vitaminol 53:43–47. https://doi.org/10.3177/jnsv.53.43
Peters V, Spangenberg B (2019) Equol determination in cattle manure by HPTLC-DART-TOF-MS. J Liq Chromatogr Relat Technol 42:311–316. https://doi.org/10.1080/10826076.2019.1585616
Xu X, Wang H, Murphy PA, Cook L, Hendrich S (1994) Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr 124:825–832. https://doi.org/10.1093/jn/124.6.825
Setchell KD, Clerici C (2010) Equol: history, chemistry, and formation. J Nutr 140:1355S-1362S. https://doi.org/10.3945/jn.109.119776
Bonorden MJ, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS (2004) Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr 58:1635–1642. https://doi.org/10.1038/sj.ejcn.1602020
Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W (2007) Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol 61:372–383. https://doi.org/10.1111/j.1574-6941.2007.00330.x
Ward WE, Kim S, Chan D, Fonseca D (2005) Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains. J Nutr Biochem 16:743–749. https://doi.org/10.1016/j.jnutbio.2005.04.002
Mayo B, Vázquez L, Flórez AB (2019) Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 11:2231. https://doi.org/10.3390/nu11092231
Fritz H, Seely D, Flower G, Skidmore B, Fernandes R, Vadeboncoeur S, Kennedy D, Cooley K, Wong R, Sagar S, Sabri E, Fergusson D (2013) Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS ONE 8:e81968. https://doi.org/10.1371/journal.pone.0081968
Yoshikata R, Yar Myint KZ, Ohta H (2018) Effects of equol supplement on bone and cardiovascular parameters in middle-aged japanese women: a prospective observational study. J Altern Complement Med 24:701–708. https://doi.org/10.1089/acm.2018.0050
Acknowledgements
The authors are very thankful to the Indian Council Medical Research (ICMR), New Delhi, Ministry of Health and Family Welfare, Government of India for providing financial support to this project under grant sanction no. 3/1/2/89/2018-(Nut).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We would like to declare that this research work did not involve any conflict of interest.
Ethical approval
The authors declare that the animals used in this research work are approved by the Institutional Animal Ethics committee (IAEC), Jamia Hamdard registration number 173/GO/Re/S/2000/CPCSEA project number 1476, and the animals were used per CPCSEA Guidelines for animal experimentation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gangwar, M., Khan, J., Alam, M.S. et al. Development of a high-performance thin-layer chromatography method for the rapid quantification of S-equol in biological samples of albino Wistar rats. JPC-J Planar Chromat 37, 57–67 (2024). https://doi.org/10.1007/s00764-024-00287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-024-00287-y