Abstract
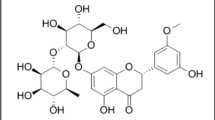
Two validated, simple and precise densitometric high-performance thin-layer chromatography (HPTLC) quantification methods were proposed for both qualitative and quantitative estimation of oleuropein in Olea europaea leaves and a pharmaceutical product utilizing normal-phase and reversed-phase silica gel TLC plates. In method I, 10 × 20 cm glass plates coated with 0.2 mm thin layers of normal-phase silica gel 60 containing F254 (E-Merck, Germany) and a mixture of ethyl acetate‒methanol‒water (8:1:0.5, V/V) were used as the stationary and the mobile phase, respectively. Method II utilized 10 × 20 cm glass-backed plates supporting 0.2 mm layers of RP-18 silica gel 60 containing F254 (E-Merck, Germany) as the stationary phase and green solvents mixture composed of ethanol‒water (5.5:4.5, V/V) as the mobile phase. The two methods resulted in sharp, symmetrical, well-resolved peaks at RF values of 0.47 ± 0.02 and 0.78 ± 0.03 with linearity ranges 200‒1400 ng/spot (r2 = 0.9994) and 200‒1400 ng/spot (r2 = 0.9996) for method I and method II, respectively. Spots corresponding to oleuropein were scanned at 200 nm. The two methods complied with the ICH guidelines for validation. Due to simplicity, low cost and short analysis time, the methods can be good alternatives for the quality control of different products containing olive leaves extract or pure oleuropein.






Similar content being viewed by others
Availability of data and material
Data are available with no restriction.
Change history
04 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00764-021-00100-0
References
Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS (2003) Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26:1277–1294
Komaki E, Yamaguchi S, Kinoshita M, Kakehi K, Ohta Y, Tsukada Y (2003) Identification of anti-α-amylase components from olive leaf extracts. Food Sci Tech Res 9:35–39
Wainstein J, Ganz T, Boaz M, Bar Dayan Y, Dolev E, Kerem Z, Madar Z (2012) Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food 15:605–610
Sarbishegi M, Gorgich EAC, Khajavi O (2017) Olive leaves extract improved sperm quality and antioxidant status in the testis of rat exposed to rotenone. Nephro-Urol Mon 9(3):e47127
Candar A, Demirci H, Baran AK, Akpınar Y (2018) The association between quality of life and complementary and alternative medicine use in patients with diabetes mellitus. Complement Ther Clin Pract 31:1–6
Soliman GA, Saeedan AS, Abdel-Rahman RF, Ogaly HA, Abd-Elsalam RM, Abdel-Kader MS (2019) Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: molecular and biochemical study. Saudi Pharm J 27:326–340
Cumaoglu A, Rackova L, Stefek M, Kartal M, Maechler P, Karasu C (2011) Effects of olive leaf polyphenols against H2O2 toxicity in insulin secreting β-cells. Acta Biochim Pol 58:45–50
Cardoso SM, Falcao SI, Peres AM, Domingues MR (2011) Oleuropein/ligstroside isomers and their derivatives in Portuguese olive mill wastewaters. Food Chem 129:291–296
Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N (2012) Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci 13:3291–3340
Boka V-I, Argyropoulou A, Gikas E, Angelis A, Aligiannis N, Skaltsounis A-L (2015) Employment of high-performance thin-layer chromatography for the quantification of oleuropein in olive leaves and the selection of a suitable solvent system for its isolation with centrifugal partition chromatography. Planta Med 81:1628–1635
Czerwińska ME, Ziarek M, Bazylko A, Osińska E, Kiss AK (2015) Quantitative determination of secoiridoids and phenylpropanoids in different extracts of Ligustrum vulgare L. leaves by a validated HPTLC-photodensitometry method. Phytochem Anal 26:253–260
ICH (2005) International conference on harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use, harmonised triplicate guideline on validation of analytical procedures: text and methodology Q2 (R1), complementary guideline on methodology incorporated in November 2005 by the ICH steering committee, IFPMA, Geneva
Convention USP (2010) United states pharmacopoia 38-national formulary 33. Stationery Office, Rockville
British Pharmacopoeia Commission (2013) British pharmacopoeia, 13th edn. Stationery Office, Great Britain
Acknowledgements
This project was supported by the Research Grant from the Deanship of Scientific Research, Prince Sattam Bin Abdulaziz University, Al-Kharj, Kingdom of Saudi Arabia (Grant No. 2020/03/17308).
Funding
Deanship of Scientific Research, Prince Sattam Bin Abdulaziz University, Al-Kharj, Kingdom of Saudi Arabia (Grant No. 2020/03/17308).
Author information
Authors and Affiliations
Contributions
PA was involved in funding acquisition, methodology, investigation, validation; MTA helped in resources and investigation; HHZ contributed to resources, validation, methodology, conceptualization, writing-reviewing and editing; MHA was involved in validation, methodology, writing-original draft preparation; MSA-K helped in data curation, visualization, supervision, conceptualization, writing-reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Additional information
The original online version of this article was revised: The correct spelling of the co-author's surname is Zaatout.
Rights and permissions
About this article
Cite this article
Alam, P., Alanazi, M.T., Zattout, H.H. et al. Densitometric high-performance thin-layer chromatography methods for the quantification of oleuropein in Olea europaea leaves and pharmaceutical preparation utilizing normal- and reversed-phase silica gel plates. JPC-J Planar Chromat 33, 609–616 (2020). https://doi.org/10.1007/s00764-020-00066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-020-00066-5