Summary.
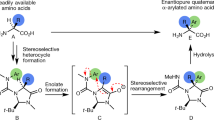
An efficient and easily applicable method for the synthesis of a variety of hydroxy-α-amino acids analogues of serine and phenylalanine has been established. The method involves the stereoselective intramolecular conjugate addition of the benzamide group to cyclohexenone promoted by Lewis acid. Subsequent transformations of functional groups pro-vide the conformationally constrained 2-hydroxy- and 2,4-dihydroxy-6-phenylcyclohexane-α-amino acids.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received February 5, 1999, Accepted May 16, 1999
Rights and permissions
About this article
Cite this article
Avenoza, A., Busto, J., Cativiela, C. et al. Synthesis of conformationally constrained hydroxy-α-amino acids by intramolecular conjugate addition. Amino Acids 18, 117–127 (2000). https://doi.org/10.1007/s007260050010
Issue Date:
DOI: https://doi.org/10.1007/s007260050010