Abstract
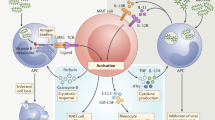
An important subtype of the innate-like T lymphocytes is mucosal-associated invariant T (MAIT) cells expressing a semi-invariant T cell receptor α (TCR-α) chain. MAIT cells could be activated mainly by TCR engagement or cytokines. They have been found to have essential roles in various immune mediated. There have been growing preclinical and clinical findings that show an association between MAIT cells and the physiopathology of inflammatory bowel diseases (IBD). Of note, published reports demonstrate contradictory findings regarding the role of MAIT cells in IBD patients. A number of reports suggests a protective effect, whereas others show a pathogenic impact. The present review article aimed to explore and discuss the findings of experimental and clinical investigations evaluating the effects of MAIT cells in IBD subjects and animal models. Findings indicate that MAIT cells could exert opposite effects in the course of IBD, including an anti-inflammatory protective effect of blood circulating MAIT cells and an effector pathogenic effect of colonic MAIT cells. Another important finding is that blood levels of MAIT cells can be considered as a potential biomarker in IBD patients.

Similar content being viewed by others
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abraham C (2009) “ChoJH.” Inflammatory bowel disease. N Engl J Med 361:2066–2078
Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, Carassiti D, Reynolds R, Salvetti M, Calabresi PA (2013) Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136(9):2888–2903
Awad W, Ler GJM, Xu W, Keller AN, Mak JYW, Lim XY, Liu L, Eckle SBG, Le Nours J, McCluskey J (2020) The molecular basis underpinning the potency and specificity of MAIT cell antigens. Nat Immunol 21(4):400–411
Böttcher K, Rombouts K, Saffioti F, Roccarina D, Rosselli M, Hall A, Luong T, Tsochatzis EA, Thorburn D, Pinzani M (2018) MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 68(1):172–186
Brand S (2009) Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 58(8):1152–1167
Chen Z, Wang H, D’Souza C, Sun S, Kostenko L, Eckle SBG, Meehan BS, Jackson DC, Strugnell RA, Cao H (2017) Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol 10(1):58–68
Chiba A, Tajima R, Tomi C, Miyazaki Y, Yamamura T, Miyake S (2012) Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum 64(1):153–161
Chiba A, Tamura N, Yoshikiyo K, Murayama G, Kitagaichi M, Yamaji K, Takasaki Y, Miyake S (2017) Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res Ther 19(1):1–10
Chua W-J, Hansen TH (2012) Vitamins prime immunity. Nature 491(7426):680–681
Chua W-J, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH (2012) Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 80(9):3256–3267
Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H (2014) T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509(7500):361–365
Corridoni D, Antanaviciute A, Gupta T, Fawkner-Corbett D, Aulicino A, Jagielowicz M, Parikh K, Repapi E, Taylor S, Ishikawa D (2020) Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med 26(9):1480–1490
Cosgrove C, Ussher JE, Rauch A, Gärtner K, Kurioka A, Hühn MH, Adelmann K, Kang Y-H, Fergusson JR, Simmonds P (2013) Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 121(6):951–961
Croxford JL, Miyake S, Huang Y-Y, Shimamura M, Yamamura T (2006) Invariant Vα19i T cells regulate autoimmune inflammation. Nat Immunol 7(9):987–994
Dou Y, Maurer K, Conrad M, Patel T, Shraim R, Sullivan KE, Kelsen J (2021) Mucosal invariant T cells are diminished in very early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr 73(4):529–536
Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood 117(4):1250–1259
Eberhard JM, Hartjen P, Kummer S, Schmidt RE, Bockhorn M, Lehmann C, Balagopal A, Hauber J, Van Lunzen J, Zur Wiesch JS (2014) CD161+ MAIT cells are severely reduced in peripheral blood and lymph nodes of HIV-infected individuals independently of disease progression. PLoS ONE 9(11):e111323
Eckle SBG, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HEG, Reantragoon R, Chen Z, Gherardin NA, Beddoe T, Liu L (2014) A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med 211(8):1585–1600
Flament H, Rouland M, Beaudoin L, Toubal A, Bertrand L, Lebourgeois S, Rousseau C, Soulard P, Gouda Z, Cagninacci L (2021) Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat Immunol 22(3):322–335
Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS (2004) Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Investig 113(10):1490–1497
Fuss IJ, Strober W (2008) The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol 1(1):S31–S33
Giuffrida P, Corazza GR, Di Sabatino A (2018) Old and new lymphocyte players in inflammatory bowel disease. Dig Dis Sci 63:277–288
Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua W-J, Yu YYL (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8(6):e1000407
Gracey E, Qaiyum Z, Almaghlouth I, Lawson D, Karki S, Avvaru N, Zhang Z, Yao Y, Ranganathan V, Baglaenko Y (2016) IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis 75(12):2124–2132
Haga K, Chiba A, Shibuya T, Osada T, Ishikawa D, Kodani T, Nomura O, Watanabe S, Miyake S (2016) MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J Gastroenterol Hepatol 31(5):965–972
Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ (2015) Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal Immunol 8(6):1226–1236
Hayashi E, Chiba A, Tada K, Haga K, Kitagaichi M, Nakajima S, Kusaoi M, Sekiya F, Ogasawara M, Yamaji K (2016) Involvement of mucosal-associated invariant T cells in ankylosing spondylitis. J Rheumatol 43(9):1695–1703
Hiejima E, Kawai T, Nakase H, Tsuruyama T, Morimoto T, Yasumi T, Taga T, Kanegane H, Hori M, Ohmori K (2015) Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm Bowel Dis 21(7):1529–1540
Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, Meehan BS, Kostenko L, Turner SJ, Corbett AJ (2019) Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep 28(12):3249–3262
Hinks TSC, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH (2015) Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol 136(2):323–333
Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PDR, Wehkamp J, Feagan BG, Yao MD, Karczewski M (2012) Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61(12):1693–1700
Illés Z, Shimamura M, Newcombe J, Oka N, Yamamura T (2004) Accumulation of Vα7. 2–Jα33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol 16(2):223–230
Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, Su J, Zhai F, Wang R, Zhang G (2014) Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med 190(3):329–339
Jo J, Tan AT, Ussher JE, Sandalova E, Tang X-Z, Tan-Garcia A, To N, Hong M, Chia A, Gill US (2014) Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 10(6):e1004210
Ju JK, Cho Y-N, Park K-J, Kwak HD, Jin H-M, Park S-Y, Kim HS, Kee S-J, Park Y-W (2020) Activation, deficiency, and reduced IFN-γ production of mucosal-associated invariant T cells in patients with inflammatory bowel disease. J Innate Immun 12(5):422–434
Kang S-J, Jin H-M, Won EJ, Cho Y-N, Jung H-J, Kwon Y-S, Kee HJ, Ju JK, Kim J-C, Kim UJ (2016) Activation, impaired tumor necrosis factor-α production, and deficiency of circulating mucosal-associated invariant T cells in patients with scrub typhus. PLoS Negl Trop Dis 10(7):e0004832
Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491(7426):717–723
Koay H-F, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, Nold-Petry CA, Nold MF, Bedoui S (2016) A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol 17(11):1300–1311
Kwon Y-S, Cho Y-N, Kim M-J, Jin H-M, Jung H-J, Kang J-H, Park K-J, Kim T-J, Kee HJ, Kim N (2015) Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis 95(3):267–274
Kwon YS, Jin H-M, Cho Y-N, Kim M-J, Kang J-H, Jung H-J, Park K-J, Kee HJ, Kee S-J, Park Y-W (2016) Mucosal-associated invariant T cell deficiency in chronic obstructive pulmonary disease. COPD 13(2):196–202
Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11(8):701–708
Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ (2015) Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43(4):727–738
Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, Somsouk M, Deeks SG, Martin JN, Moll M (2013) Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121(7):1124–1135
Leng T, Akther HD, Hackstein C-P, Powell K, King T, Friedrich M, Christoforidou Z, McCuaig S, Neyazi M, Arancibia-Cárcamo CV (2019) TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep 28(12):3077–3091
Loh L, Wang Z, Sant S, Koutsakos M, Jegaskanda S, Corbett AJ, Liu L, Fairlie DP, Crowe J, Rossjohn J (2016) Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc Natl Acad Sci 113(36):10133–10138
Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Da Silva J, Allatif O, Rossjohn J (2015) Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Investig 125(4):1752–1762
Mak JYW, Liu L, Fairlie DP (2021) Chemical modulators of mucosal associated invariant T cells. Acc Chem Res 54(17):3462–3475
Mak JYW, Xu W, Reid RC, Corbett AJ, Meehan BS, Wang H, Chen Z, Rossjohn J, McCluskey J, Liu L (2017) Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat Commun 8(1):14599
Matsuyama H, Isshiki T, Chiba A, Yamaguchi T, Murayama G, Akasaka Y, Eishi Y, Sakamoto S, Homma S, Miyake S (2019) Activation of mucosal-associated invariant T cells in the lungs of sarcoidosis patients. Sci Rep 9(1):13181
Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T (2011) Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol 23(9):529–535
Murayama G, Chiba A, Suzuki H, Nomura A, Mizuno T, Kuga T, Nakamura S, Amano H, Hirose S, Yamaji K (2019) A critical role for mucosal-associated invariant T cells as regulators and therapeutic targets in systemic lupus erythematosus. Front Immunol 10:2681
Nakajima S, Chiba A, Makiyama A, Hayashi E, Murayama G, Yamaji K, Kobayashi S, Tamura N, Takasaki Y, Miyake S (2020) Association of mucosal-associated invariant T cells with different disease phases of polymyalgia rheumatica. Rheumatology 59(10):2939–2946
Napier RJ, Adams EJ, Gold MC, Lewinsohn DM (2015) The role of mucosal associated invariant T cells in antimicrobial immunity. Front Immunol 6:344
O’Connor W Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA (2009) A protective function for interleukin 17A in T cell–mediated intestinal inflammation. Nat Immunol 10(6):603–609
O’Connor W Jr, Zenewicz LA, Flavell RA (2010) The dual nature of TH17 cells: shifting the focus to function. Nat Immunol 11(6):471–476
Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y (2004) Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 110(1):55–62
Park D, Kim HG, Kim M, Park T, Ha H-H, Lee DH, Park K-S, Park SJ, Lim HJ, Lee CH (2019) Differences in the molecular signatures of mucosal-associated invariant T cells and conventional T cells. Sci Rep 9(1):1–10
Patel O, Kjer-Nielsen L, Le Nours J, Eckle SBG, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B (2013) Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 4(1):2142
Porcelli S, Yockey CE, Brenner MB, Balk SP (1993) Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8-alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 178(1):1–16
Provine NM, Amini A, Garner LC, Spencer AJ, Dold C, Hutchings C, Silva Reyes L, FitzPatrick MEB, Chinnakannan S, Oguti B (2021) MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 371(6528):521–526
Ratcliffe MJH (2016) Encyclopedia of immunobiology. Academic Press, New York
Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SBG, Uldrich AP, Birkinshaw RW, Patel O (2013) Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 210(11):2305–2320
Rouxel O, Da Silva J, Beaudoin L, Nel I, Tard C, Cagninacci L, Kiaf B, Oshima M, Diedisheim M, Salou M (2017) Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol 18(12):1321–1331
Salou M, Legoux F, Gilet J, Darbois A, du Halgouet A, Alonso R, Richer W, Goubet A-G, Daviaud C, Menger L (2019) A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J Exp Med 216(1):133–151
Serriari N-E, Gondois-Rey F, Guillaume Y, Remmerswaal E, Pastor S, Messal N, Truneh A, Hirsch I, van Lier RAW, Olive D (2010) B and T lymphocyte attenuator is highly expressed on CMV-specific T cells during infection and regulates their function. J Immunol 185(6):3140–3148
Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O (2014) Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 176(2):266–274
Solders M, Gorchs L, Erkers T, Lundell A-C, Nava S, Gidlöf S, Tiblad E, Magalhaes I, Kaipe H (2017) MAIT cells accumulate in placental intervillous space and display a highly cytotoxic phenotype upon bacterial stimulation. Sci Rep 7(1):1–13
Tilloy F, Treiner E, Park S-H, Garcia C, Lemonnier F, De La Salle H, Bendelac A, Bonneville M, Lantz O (1999) An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib–restricted α/β T cell subpopulation in mammals. J Exp Med 189(12):1907–1921
Tominaga K, Yamagiwa S, Setsu T, Kimura N, Honda H, Kamimura H, Honda Y, Takamura M, Yokoyama J, Suzuki K (2017) Possible involvement of mucosal-associated invariant T cells in the progression of inflammatory bowel diseases. Biomed Res 38(2):111–121
Tomkovich S, Jobin C (2016) Microbiota and host immune responses: a love–hate relationship. Immunology 147(1):1–10
Touch S, Assmann KE, Aron-Wisnewsky J, Marquet F, Rouault C, Fradet M, Mosbah H, Consortium M, Isnard R, Helft G (2018) Mucosal-associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. FASEB J 32(9):5078–5089
Treiner E (2015) Mucosal-associated invariant T cells in inflammatory bowel diseases: bystanders, defenders, or offenders? Front Immunol 6:27
Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422(6928):164–169
Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P (2014) CD 161++ CD 8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+ IL-18 in a TCR-independent manner. Eur J Immunol 44(1):195–203
van Wilgenburg B, Loh L, Chen Z, Pediongco TJ, Wang H, Shi M, Zhao Z, Koutsakos M, Nüssing S, Sant S (2018) MAIT cells contribute to protection against lethal influenza infection in vivo. Nat Commun 9(1):4706
Van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, De Lara C, Cole S, Vasanawathana S, Limpitikul W (2016) MAIT cells are activated during human viral infections. Nat Commun 7(1):11653
Varelias A, Bunting MD, Ormerod KL, Koyama M, Olver SD, Straube J, Kuns RD, Robb RJ, Henden AS, Cooper L (2018) Recipient mucosal-associated invariant T cells control GVHD within the colon. J Clin Investig 128(5):1919–1936
Wallace KL, Zheng L-B, Kanazawa Y, Shih DQ (2014) Immunopathology of inflammatory bowel disease. World J Gastroenterol 20(1):6
Wang JJ, Macardle C, Weedon H, Beroukas D, Banovic T (2016) Mucosal-associated invariant T cells are reduced and functionally immature in the peripheral blood of primary Sjögren’s syndrome patients. Eur J Immunol 46(10):2444–2453
Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, Piedavent M, Friese MA (2014) CD 8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol 44(10):3119–3128
Won EJ, Ju JK, Cho Y-N, Jin H-M, Park K-J, Kim T-J, Kwon Y-S, Kee HJ, Kim J-C, Kee S-J (2016) Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer. Oncotarget 7(46):76274
Yasutomi Y, Chiba A, Haga K, Murayama G, Makiyama A, Kuga T, Watanabe M, Okamoto R, Nagahara A, Nagaishi T (2022) Activated mucosal-associated invariant T cells have a pathogenic role in a murine model of inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 13(1):81–93
Zhang C, Zhang J, Niu J, Zhang J, Tian Z (2008a) Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine 42(1):128–136
Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z (2008b) Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol 69(8):490–500
Funding
This study was supported by Project of Shanghai New District Health Commission (PW2021A-25).
Author information
Authors and Affiliations
Contributions
QI contributed to the conception and design of the study. LW and ZC performed data acquisition and prepared the first draft. QI, LW, and ZC performed revised the draft critically for important intellectual content. All the authors read and approved the final version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling editor: S. Broer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Chen, Z. & Lv, Q. Mucosal-associated invariant T cells display both pathogenic and protective roles in patients with inflammatory bowel diseases. Amino Acids 55, 1819–1827 (2023). https://doi.org/10.1007/s00726-023-03344-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-023-03344-8