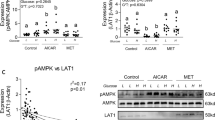
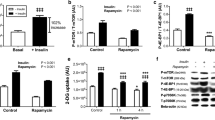
Abstract
Previous work has shown that dietary l-arginine (Arg) supplementation reduced white fat mass in obese rats. The present study was conducted with cell models to define direct effects of Arg on energy-substrate oxidation in hepatocytes, skeletal muscle cells, and adipocytes. BNL CL.2 mouse hepatocytes, C2C12 mouse myotubes, and 3T3-L1 mouse adipocytes were treated with different extracellular concentrations of Arg (0, 15, 50, 100 and 400 µM) or 400 µM Arg + 0.5 mM NG-nitro-l-arginine methyl ester (L-NAME; an NOS inhibitor) for 48 h. Increasing Arg concentrations in culture medium dose-dependently enhanced (P < 0.05) the oxidation of glucose and oleic acid to CO2 in all three cell types, lactate release from C2C12 cells, and the incorporation of oleic acid into esterified lipids in BNL CL.2 and 3T3-L1 cells. Arg at 400 µM also stimulated (P < 0.05) the phosphorylation of AMP-activated protein kinase (AMPK) in all three cell types and increased (P < 0.05) NO production in C2C12 and BNL CL.2 cells. The inhibition of NOS by L-NAME moderately reduced (P < 0.05) glucose and oleic acid oxidation, lactate release, and the phosphorylation of AMPK and acetyl-CoA carboxylase (ACC) in BNL CL.2 cells, but had no effect (P > 0.05) on these variables in C2C12 or 3T3-L1 cells. Collectively, these results indicate that Arg increased AMPK activity and energy-substrate oxidation in BNL CL.2, C2C12, and 3T3-L1 cells through both NO-dependent and NO-independent mechanisms.










Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- AU:
-
Arbitrary unit
- CPT-I:
-
Carnitine palmitoyltransferase I
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- KHB:
-
Krebs–Henseleit bicarbonate
- MCD:
-
Malonyl-CoA decarboxylase
- L-NAME:
-
NG-Nitro-l-arginine methyl ester
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- SCD-1:
-
Stearoyl-CoA desaturase 1
- SDS:
-
Sodium dodecyl sulfate
- S6K1:
-
Ribosomal protein S6 kinase beta-1
- TTBS:
-
Tris–Tween-buffered saline
References
Alam MA, Kauter K, Withers K, Sernia C, Brown L (2013) Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct 4:83–91
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Assaad H, Zhou L, Carroll RJ, Wu G (2014) Rapid publication-ready MS word tables for one-way ANOVA. Springer plus 3:474
Bergen W, Mersmann HJ (2005) Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr 135:2499–2502
Boon MR, Hanssen MJW, Brans B, Hülsman CJM, Hoeks J, Nahon KJ, Bakker C, van Klinken JB, Havekes B, Schaart G, Jazet IM, Rensen PCN, van Marken Lichtenbelt WD (2019) Effect of l-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men: a randomised double-blinded crossover study. Diabetologia 62:112–122
Ceddia RB, William WN Jr, Lima FB, Flandin P, Curi R, Giacobino JP (2000) Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem 267:5952–5958
Wu G, Jaeger LA, Bazer FW, Rhoads JM (2004b) Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 15:442–451
Chen JQ, Ma XS, Yang Y, Dai ZL, Wu ZL, Wu G (2018) Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 50(5):629–640. https://doi.org/10.1007/s00726-018-2537-3
Closs EI, Boissel JP, Habermeier A, Rotmann A (2006) Structure and function of cationic amino acid transporters (CATs). J Membr Biol 213:67–77
Dai ZL, Wu ZL, Yang Y, Wang JJ, Satterfield MC, Meininger CJ, Bazer FW, Wu G (2013) Nitric oxide and energy metabolism in mammals. BioFactors 39:383–391
Denninger JW, Marletta MA (1999) Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1411:334–350
Dole VP, Meinertz H (1960) Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem 235:2595–2599
Durante W (2020) Amino acids in circulatory function and health. Adv Exp Med Biol 1265:39–56
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837
Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H (1994) Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23:1121–1131
Fouad AM, El-Senousey HK, Yang XJ, Yao JH (2013) Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal 7:1239–1245
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135:714–721
Wu G, Bazer FW, Cudd TA, Jobgen WS, Kim SW, Lassala A, Li P, Matis JH, Meininger CJ, Spencer TE (2007) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S-1680S
Garcia-Villafranca J, Guillen A, Castro J (2003) Involvement of nitric oxide/cyclic GMP signaling pathway in the regulation of fatty acid metabolism in rat hepatocytes. Biochem Pharmacol 65:807–812
He WL, Wu G (2022) Oxidation of amino acids, glucose, and fatty acids as metabolic fuels in enterocytes of developing pigs. Amino Acids 54(7):1025–1039. https://doi.org/10.1007/s00726-022-03151-7
Houdijk APJ, Visser JJ, Rijinsburger ER, Teerlink T, van Leeuwen PAM (1998) Dietary glutamine supplementation reduces plasma nitrate levels in rats. Clin Nutr 17:11–14
Jobgen WS (2007) Dietary L-arginine supplementation reduces fat mass in diet-induced obese rats. PhD Dissertation. Texas A&M University, College Station, TX, USA
Jobgen WS, Wu G (2022) Dietary L-arginine supplementation increases the hepatic expression of AMP-activated protein kinase in rats. Amino Acids. https://doi.org/10.1007/s00726-022-03194-w
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Jobgen WS, Jobgen SC, Li H, Meininger CJ, Wu G (2007) Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. J Chromatogr B 851:71–82
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Kahn BB, Alquier T, Carling D, Hardie DG (2005a) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
Kahn R, Buse J, Ferranini E, Stern M (2005b) The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 48:1684–1699
Kalupahana NS, Claycombe KJ, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N (2010) Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr 140:1915–1922
Khosroshahi MZ, Asbaghi O, Moradi S, Kelishadi MR, Kaviani M, Mardani M, Jalili C (2020) The effects of supplementation with L-arginine on anthropometric indices and body composition in overweight or obese subjects: a systematic review and meta-analysis. J Funct Foods 71:104022
Yan H, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M, Lau DC (2002) Nitric oxide promotes differentiation of rat white preadipocytes in culture. J Lipid Res 43:2123–2129
Kröncke KD, Kolb-Bachofen V, Berschick B, Burkart V, Kolb H (1991) Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun 175:752–758
Kuppusamy P, Kim D, Soundharrajan I, Hwang I, Choi KC (2021) Adipose and muscle cell co-culture system: A novel in vitro tool to mimic the in vivo cellular environment. Biology 10:6
Lee BS, Kang HS, Pyun KH, Choi IP (1997) Roles of tyrosine kinases in the regulation of nitric oxide synthesis in murine liver cells: Modulation of NF-kappa B activity by tyrosine kinases. Hepatology 25(4):913–919. https://doi.org/10.1002/hep.510250421
Li H, Meininger CJ, Wu G (2000) Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B 746:199–207
Li SL, Zhang YC, Liu N, Chen JQ, Guo LN, Dai ZL, Wang C, Wu ZL, Wu G (2020) Dietary L-arginine supplementation reduces lipid accretion by regulating fatty acid metabolism in Nile tilapia (Oreochromis niloticus). J Anim Sci Biotechnol 11:82
Mariotti F (2020) Arginine supplementation and cardiometabolic risk. Curr Opin Clin Metab Care 23:29–34
McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G (2010) Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids 39:349–357
McNeal CJ, Meininger CJ, Wilborn CD, Tekwe CD, Wu G (2018) Safety of dietary supplementation with arginine in adult humans. Amino Acids 50:1215–1229
Meininger CJ, Wu G (1997) l-Glutamine inhibits nitric oxide synthesis in bovine venular endothelial cells. J Pharmacol Exp Ther 281:448–453
Miczke A, Suliburska J, Pupek-Musialik D, Ostrowska L, Jabłecka A, Krejpcio Z, Skrypnik D, Bogdański P (2015) Original Article Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. Int J Clin Exp Med 8:10358–10366
Morita T, Shimada Y, Ueki H, Kanagawa A (1996) Stimulation of nitric oxide-cyclic GMP pathway by L-arginine increases the release of hepatic lipase from cultured rat hepatocytes. Biol Pharm Bull 19:1371–1373
Muller EA, Danner DJ (2004) Tissue-specific translation of murine branched-chain α-ketoacid dehydrogenase kinase mRNA is dependent upon an upstream open reading frame in the 5’-untranslated region. J Biol Chem 279:44645–44655
Nicholls-Grzemski FA, Tirmenstein MA, Fariss MW (1999) Time-dependent production of nitric oxide by rat hepatocyte suspensions. Biochem Pharmacol 57:1223–1226
Oliveira MM, Vaughan M (1964) Incorporation of fatty acids into phospholipids of erythrocyte membranes. J Lipid Res 5:156–162
Peyton KJ, Liu XM, Shebib AR, Johnson FK, Johnson RA, Durante W (2018) Arginase inhibition prevents the development of hypertension and improves insulin resistance in obese rats. Amino Acids 50:747–754
Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL (1998) Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell 95:939–950
Reid KM, Tsung A, Kaizu T, Jeyabalan G, Ikeda A, Shao L, Wu G, Murase N, Geller DA (2007) Liver I/R Injury is improved by the arginase inhibitor, N-ω-Hydroxy-Nor-L-arginine (Nor-NOHA). Am J Physiol 292:G512-517
Rhoads JM, Corl BA, Harrell R, Niu X, Gatlin L, Phillips O, Blikslager A, Moeser A, Wu G, Odle J (2007) Intestinal ribosomal p70s6k signaling is increased in piglet rotavirus enteritis. Am J Physiol 292:G913-922
Ruderman N, Prentki M (2004) AMP kinase and malonyl-CoA targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351
San Martín A, Arce-Molina R, Galaz A, Pérez-Guerra G, Felipe Barros L (2017) Nanomolar nitric oxide concentrations quickly and reversibly modulate astrocytic energy metabolism. J Biol Chem 292:9432–9438
Sansbury BE, Hill BG (2014) Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 73:383–399
Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 43:1593–1603
Schuman EM, Madison DV (1991) A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 254:1503–1506
Sellmann C, Degen C, Jin CJ, Nier A, Engstler AJ, Alkhatib DH, De Bandt J-P, Bergheim I (2017) Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 49:1215–1225
Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G (2004) Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys 41:415–433
Szlas A, Kurek JM, Krejpco Z (2022) The potential of L-arginine in prevention and treatment of disturbed carbohydrate and lipid metabolism—a review. Nutrients 14:961
Tan B, Yin Y, Liu Z, Tang W, Xu H, Kong X, Xi L, Yao K, Gu W, Smith SB, Wu G (2011) Dietary L-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem 22:441–445
Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F (2006) Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol 574:41–53
Wang YX, Watford M (2007) Glutamine, insulin and glucocorticoids regulate glutamine synthetase expression in C2C12 myotubes, Hep G2 hepatoma cells and 3T3 L1 adipocytes. Biochim Biophys Acta 1770:594–600
Wang JJ, Chen LX, Li DF, Yin YL, Wang XQ, Li P, Dangott LJ, Hu WX, Wu G (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver and skeletal muscle in newborn pigs. J Nutr 138:60–66
Williams G, Brown T, Becker L, Prager M, Giroir BP (1994) Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol 267:R1020-1025
Wu G (2022) Amino acids: biochemistry and nutrition, 2nd edn. CRC Press, Boca Raton
Wu G, Meininger CJ (2002) Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr 22:61–86
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Thompson JR (1990) The effect of glutamine on protein turnover in chick skeletal muscle in vitro. Biochem J 265:593–598
Wu G, Knabe DA, Yan W, Flynn NE (1995) Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol 268:R334-342
Wu G, Bazer FW, Tuo W, Flynn SP (1996) Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol Reprod 54:1261–1265
Wu G, Pond WG, Ott T, Bazer FW (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128:894–902
Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK (1999) Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr 129:1347–1354
Wu G, Haynes TE, Li H, Yan W, Meininger CJ (2001) Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J 353:245–252
Wu G, Knabe DA, Kim SW (2004a) Arginine nutrition in neonatal pigs. J Nutr 134:2783S-2790S
Acknowledgements
We thank Dr. Stephen B. Smith and Dr. Cynthia J. Meininger for helpful discussions, as well as Mr. Scott Jobgen for assistance in laboratory analyses of metabolites. This research was supported by grants from American Heart Association–Texas (#0755024Y and 10GRNT4480020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
Established cell lines available from commercial companies were used for this study. Thus, the present work did not require the approval for the use of animals by Institutional Animal Care and Use Committee of Texas A&M University.
Informed consent
No informed consent is required for this study.
Additional information
Handling editor: E. Ildicho Closs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jobgen, W.S., Wu, G. l-Arginine increases AMPK phosphorylation and the oxidation of energy substrates in hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids 54, 1553–1568 (2022). https://doi.org/10.1007/s00726-022-03195-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03195-9