Abstract
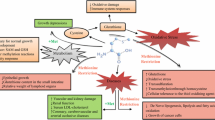
The goal of this study was to elucidate the molecular mechanisms responsible for the anti-obesity effect of L-arginine supplementation in diet-induced obese rats. Male Sprague–Dawley rats were fed either a low-fat or high-fat diet for 15 weeks. Thereafter, lean or obese rats were pair-fed their same respective diets and received drinking water containing either 1.51% L-arginine-HCl or 2.55% L-alanine (isonitrogenous control) for 12 weeks. Gene and protein expression of key enzymes in the metabolism of energy substrates were determined using real-time polymerase-chain reaction and western blotting techniques. The mRNA levels of hepatic fatty acid synthase and stearoyl-CoA desaturase were reduced (P < 0.05) but those of hepatic AMP-activated protein kinase-α (AMPKα), peroxisome proliferator activator receptor γ coactivator-1α, and carnitine palmitoyltransferase I (CPT-I), as well as skeletal muscle CPT-I were increased (P < 0.05) by L-arginine treatment. The protein expression and activity of hepatic AMPKα markedly increased (P < 0.05) but the activity of hepatic acetyl-CoA carboxylase (ACC) decreased (P < 0.05) in response to dietary L-arginine supplementation. Collectively, our results indicate that liver is the major target for the action of dietary L-arginine supplementation on reducing white-fat mass in diet-induced obese rats by inhibiting fatty acid synthesis and increasing fatty acid oxidation via the AMPK-ACC signaling pathway. Additionally, increased CPT-I expression in skeletal muscle may also contribute to the enhanced oxidation of long-chain fatty acids in L-arginine-supplemented rats.









Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- Arg:
-
L-Arginine
- AU:
-
Arbitrary unit
- BAT:
-
Brown adipose tissue
- CPT-I:
-
Carnitine palmitoyltransferase I
- DIO:
-
Diet-induced obesity
- EDL:
-
Extensor digitorum longus
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT:
-
Glucose transporter
- MCD:
-
Malonyl-CoA decarboxylase
- NOS:
-
Nitric oxide synthase
- PGC-1α:
-
Peroxisome proliferator activator receptor γ coactivator-1 α
- PKB:
-
Protein kinase B
- RP:
-
Retroperitoneal
- RT-PCR:
-
Real-time polymerase-chain reaction
- SCD-1:
-
Stearoyl-CoA desaturase-1
- SDS:
-
Sodium dodecyl sulfate
- S6K1:
-
Ribosomal protein S6 kinase beta-1
- TTBS:
-
Tris-Tween buffered saline
- UCP:
-
Uncoupling protein
References:
Alam MA, Kauter K, Withers K, Sernia C, Brown L (2013) Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct 4:83–91
Assaad H, Hou YQ, Zhou L, Carroll RJ, Wu G (2015) Rapid publication-ready MS-word tables for two-way ANOVA. Springerplus 4:33
Barber RD, Harmer DW, Coleman RA, Clark BJ (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21:389–395
Bergen W, Mersmann HJ (2005) Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr 135:2499–2502
Boon MR, Hanssen MJW, Brans B, Hülsman CJM, Hoeks J, Nahon KJ, Bakker C, van Klinken JB, Havekes B, Schaart G, Jazet IM, Rensen PCN, van Marken Lichtenbelt WD (2019) Effect of l-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men: a randomised double-blinded crossover study. Diabetologia 62:112–122
Carling D (2005) AMP-activated protein kinase: balancing the scales. Biochimie 87:87–91
Chung S, Brown JM, Sandberg MB, McIntosh M (2005) Trans-10, cis-12 CLA increases adipocyte lipolysis and alters lipid droplet-associated proteins: role of mTOR and ERK signaling. J Lipid Res 46:885–895
Dann SG, Thomas G (2006) The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 580:2821–2829
Dobrzyn A, Ntambi JM (2005) Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obes Rev 6:169–174
Durante W (2020) Amino acids in circulatory function and health. Adv Exp Med Biol 1265:39–56
Fouad AM, El-Senousey HK, Yang XJ, Yao JH (2013) Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal 7:1239–1245
Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW (2003) Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 27:875–888
Gaidhu MP, Fediuc S, Ceddia RB (2006) 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem 281:25956–25964
Garcia-Villafranca J, Guillen A, Castro J (2003) Involvement of nitric oxide/cyclic GMP signaling pathway in the regulation of fatty acid metabolism in rat hepatocytes. Biochem Pharmacol 65:807–812
Hardie DG, Carling D (1997) The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur J Biochem 246:259–273
Hayashi K, Carpenter KD, Spencer TE (2004) Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology 145:3247–3257
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19:121–135
Himms-Hagen J (2001) Does brown adipose tissue (BAT) have a role in the physiology or treatment of human obesity? Rev Endocr Metab Disord 2:395–401
Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580
Jobgen WS (2007) Dietary L-arginine supplementation reduces fat mass in diet-induced obese rats. PhD Dissertation. Texas A&M University, College Station, TX, USA
Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G (2006) Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem 17:571–588
Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee M-J, Smith SB, Spencer TE, Fried SK, Wu G (2009) Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr 139:230–237
Jobgen WS, Wu G (2022) L-Arginine increases AMPK phosphorylation and the oxidation of energy substrates in hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids. https://doi.org/10.1007/s00726-022-03195-9
Kahn BB, Alquier T, Carling D, Hardie DG (2005a) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25
Kahn R, Buse J, Ferranini E, Stern M (2005b) The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 48:1684–1699
Khosroshahi MZ, Asbaghi O, Moradi S, Kelishadi MR, Kaviani M, Mardani M, Jalili C (2020) The effects of supplementation with L-arginine on anthropometric indices and body composition in overweight or obese subjects: A systematic review and meta-analysis. J Funct Foods 71:104022
Lavau M, Fried SK, Susini C, Freychet P (1979) Mechanism of insulin resistance in adipocytes of rats fed a high-fat diet. J Lipid Res 20:8–16
Li SL, Zhang YC, Liu N, Chen JQ, Guo LN, Dai ZL, Wang C, Wu ZL, Wu G (2020) Dietary L-arginine supplementation reduces lipid accretion by regulating fatty acid metabolism in Nile tilapia (Oreochromis niloticus). J Anim Sci Biotechnol 11:82
Liu Y, Wan Q, Guan Q, Gao L, Zhao J (2006) High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem Biophys Res Commun 339:701–707
Mariotti F (2020) Arginine supplementation and cardiometabolic risk. Curr Opin Clin Metab Care 23:29–34
Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB (2006) Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281:18933–18941
McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G (2010) Beneficial effects of L-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids 39:349–357
McNeal CJ, Meininger CJ, Wilborn CD, Tekwe CD, Wu G (2018) Safety of dietary supplementation with arginine in adult humans. Amino Acids 50:1215–1229
Miczke A, Suliburska J, Pupek-Musialik D, Ostrowska L, Jabłecka A, Krejpcio Z, Skrypnik D, Bogdański P (2015) Original Article Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. Int J Clin Exp Med 8:10358–10366
Munday MR (2002) Regulation of mammalian acetyl-CoA carboxylase. Biochem Soc Trans 30:1059–1064
Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899
Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK (2002a) Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 277:32571–32577
Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW (2002b) Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol 92:2475–2482
Peyton KJ, Liu XM, Shebib AR, Johnson FK, Johnson RA, Durante W (2018) Arginase inhibition prevents the development of hypertension and improves insulin resistance in obese rats. Amino Acids 50:747–754
Puigserver P (2005) Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int J Obes (Lond) 29(Suppl 1):S5–9
Ruderman N, Prentki M (2004) AMP kinase and malonyl-CoA: target for therapy of the metabolic syndrome. Nat Med 3:340–351
San Martín A, Arce-Molina R, Galaz A, Pérez-Guerra G, Felipe Barros L (2017) Nanomolar nitric oxide concentrations quickly and reversibly modulate astrocytic energy metabolism. J Biol Chem 292:9432–9438
Sansbury BE, Hill BG (2014) Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 73:383–399
Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 43:1593–1603
Sellmann C, Degen C, Jin CJ, Nier A, Engstler AJ, Alkhatib DH, De Bandt J-P, Bergheim I (2017) Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 49:1215–1225
Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, Lopaschuk GD (2005) Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol 289:H2304-2309
Steinberg GR, Macaulay SL, Febbraio MA, Kemp BE (2006) AMP-activated protein kinase -- the fat controller of the energy railroad. Can J Physiol Pharmacol 84:655–665
Suyawan A, O’Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA (2004) Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134:24–30
Szlas A, Kurek JM, Krejpco Z (2022) The potential of L-arginine in prevention and treatment of disturbed carbohydrate and lipid metabolism–A review. Nutrients 14:961
Tan B, Yin Y, Liu Z, Tang W, Xu H, Kong X, Xi L, Yao K, Gu W, Smith SB, Wu G (2011) Dietary L-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem 22:441–445
Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F (2006) Activation of AMP-activated protein kinase in the liver: a new strategery for the management of metabolic hepatic disorders. J Physiol 574:41–53
Wang JJ, Chen LX, Li DF, Yin YL, Wang XQ, Li P, Dangott LJ, Hu WX, Wu G (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver and skeletal muscle in newborn pigs. J Nutr 138:60–66
WHO (The World Health Organization) (2016) https://www.who.int/health-topics/obesity#tab=tab_1
Wu G (2022) Amino Acids: Biochemistry and Nutrition, 2nd edn. CRC Press, Boca Raton
Wu G, Meininger CJ, McNeal CJ, Bazer FW, Rhoads JM (2021) Role of L-arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol 1332:167–187
Xiao XQ, Grove KL, Grayson BE, Smith MS (2004) Inhibition of uncoupling protein expression during lactation: role of leptin. Endocrinology 145:830–838
Yan H, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M, Lau DC (2002) Nitric oxide promotes differentiation of rat white preadipocytes in culture. J Lipid Res 43:2123–2129
Acknowledgements
We thank Dr. Stephen B. Smith and Dr. Cynthia J. Meininger for helpful discussion, as well as Mr. Scott Jobgen for laboratory analyses of metabolites. This work was supported by grants from American Heart Association–Texas (#0755024Y and #10GRNT4480020).
Funding
American Heart Association-Texas, #0755024Y, Guoyao Wu, #10GRNT4480020, Guoyao Wu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Informed consent
No informed consent is required for this study.
Additional information
Handling editor: E. Ildicho Closs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jobgen, W.S., Wu, G. Dietary L-arginine supplementation increases the hepatic expression of AMP-activated protein kinase in rats. Amino Acids 54, 1569–1584 (2022). https://doi.org/10.1007/s00726-022-03194-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03194-w