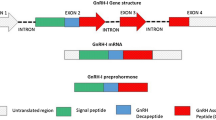
Abstract
GnRH-I and GnIH are the key neuropeptides that regulate the hypothalamic–pituitary–gonadal axis in mammals during aging. Polyamines are important aliphatic amines that are expressed in the brain and show variation with aging. The present study demonstrates evidence of variation in the level of expression of polyamines, GnRH-I and GnIH in the hypothalamus of female mice during aging. The study also suggests regulatory effects of polyamines over expression of the hypothalamic GnRH-I. The study shows a significant positive correlation between polyamines, its associated factors and GnRH-I along with significant negative correlation between polyamines, its associated factors and GnIH. This is the first study to report the effect of polyamines along with lactate or TNF-α or both on GnRH-I expression in GT1-7 cell line. TNF-α and lactate significantly decreased hypothalamic GnRH-I mRNA expression in GT1-7 cells when treated for 24 h. Polyamines (putrescine and agmatine) in contrast, significantly increased GnRH-I mRNA expression in GT1-7 cells when treated for 24 h. Also, polyamines increased GnRH-I mRNA expression when treated in presence of TNF-α or lactate thereby suggesting its neuro-protective role. This study also found 3809 differentially expressed genes through RNA-seq done between the hypothalamic GT1-7 cells treated with putrescine only versus TNF-α and putrescine. The present study suggests for the first time that putrescine treatment to TNFα-primed GT1-7 cells upregulates GnRH-I expression via regulation of several pathways such as calcium ion pathway, estrogen signaling, clock genes as well as regulating other metabolic process like neuronal differentiation and neurulation.









Similar content being viewed by others
References
Anders S, Huber W (2010) Differential expression analysis for sequence count data. In: Nature Precedings 1-1
Barabás K, Szabó-Meleg E, Ábrahám IM (2020) Effect of inflammation on female gonadotropin-releasing hormone (GnRH) neurons: mechanisms and consequences. Int J Mol Sci 21(2):529
Bauman BM, Yin W, Gore AC, Wu TJ (2017) Regulation of gonadotropin-releasing hormone-(1–5) signaling genes by estradiol is age dependent. Front Endocrinol 8:282
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (methodol) 57(1):289–300
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK (2000) Ageing, tumour necrosis factor-α (TNF-α) and atherosclerosis. Clin Exp Immunol 121(2):255–260
Cai D, Khor S (2019) “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab 30(1):19–35
Carvalho-Silva D, Pierleoni A, Pignatelli M, Ong C, Fumis L, Karamanis N, Dunham I (2019) Open targets platform: new developments and updates two years on. Nucleic Acids Res 47(D1):D1056–D1065
Chappell PE, White RS, Mellon PL (2003) Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 23(35):11202–11213
Das R, Kanungo MS (1982) Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp Gerontol 17(2):95–103
Del Portal DA, Shofer F, Mikkelsen ME, Dorsey PJ Jr, Gaieski DF, Goyal M, Pines JM (2010) Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 17(3):260–268
Dudkowska M, Lai J, Gardini G, Stachurska A, Grzelakowska-Sztabert B, Colombatto S, Manteuffel-Cymborowska M (2003) Agmatine modulates the in vivo biosynthesis and interconversion of polyamines and cell proliferation. Biochim Biophys Acta (BBA) Gen Subjects 1619(2):159–166
Ferin M, Jewelewicz R, Warren M (1993) The menstrual cycle: physiology, reproductive disorders, and infertility. Oxford University Press, Oxford
Fernandes JR, Jain S, Banerjee A (2017) Expression of ODC1, SPD, SPM and AZIN1 in the hypothalamus, ovary and uterus during rat estrous cycle. Gen Comp Endocrinol 246:9–22
Fernandes JR, Moitra A, Tsutsui K, Banerjee A (2020) Regulation of the hypothalamic GnRH–GnIH system by putrescine in adult female rats and GT1-7 neuronal cell line. J Exp Zool Part A Ecol Integr Physiol 333(4):214–229
Frankola KA, Greig NH, Luo W, Tweedie D (2011) Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets (formerly Curr Drug Targets CNS Neurol Disord) 10(3):391–403
Ganapathi M, Padgett LR, Yamada K, Devinsky O, Willaert R, Person R, Chung WK (2019) Recessive rare variants in deoxyhypusine synthase, an enzyme involved in the synthesis of hypusine, are associated with a neurodevelopmental disorder. Am J Hum Genet 104(2):287–298
Gilad GM, Gilad VH (1999) Novel polyamine derivatives as neuroprotective agents. J Pharmacol Exp Ther 291(1):39–43
Gruenewald DA, Naai MA, Marck BT, Matsumoto AM (2000) Age-related decrease in hypothalmic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to gnrh, in the male brown norway rat. J Androl 21(1):72–84
Hastings M (1998) The brain, circadian rhythms, and clock genes. BMJ 317(7174):1704–1707
Iwasa T, Matsuzaki T, Yano K, Mayila Y, Yanagihara R, Yamamoto Y, Irahara M (2018) Effects of low energy availability on reproductive functions and their underlying neuroendocrine mechanisms. J Clin Med 7(7):166
Jänne J, Raina A, Siimes M (1964) Spermidine and spermine in rat tissues at different ages. Acta Physiol Scand 62(4):352–358
Jiang D, Mo G, Jiang Y, Kang B (2021) Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus. Open Life Sci 16(1):39–45
Kavanaugh SI, Nozaki M, Sower SA (2008) Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology 149(8):3860–3869
Kim M, Jung K, Kim IS, Lee IS, Ko Y, Shin JE, Park KI (2018) TNF-α induces human neural progenitor cell survival after oxygen–glucose deprivation by activating the NF-κB pathway. Exp Mol Med 50(4):1–14
Kotagale NR, Taksande BG, Inamdar NN (2019) Neuroprotective offerings by agmatine. Neurotoxicology 73:228–245
Lefèvre PL, Palin MF, Murphy BD (2011) Polyamines on the reproductive landscape. Endocr Rev 32(5):694–712
Liu D, Mo G, Tao Y, Wang H, Liu XJ (2017) Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev 29(7):1392–1400
Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI (1990) Immortalization of hypothalamic GnRH by genetically targeted tumorigenesis. Neuron 5(1):1–10
Minois N, Carmona-Gutierrez D, Madeo F (2011) Polyamines in aging and disease. Aging (albany, N y) 3(8):716
Morrison LD, Cao XC, Kish SJ (1998) Ornithine decarboxylase in human brain: influence of aging, regional distribution, and Alzheimer’s disease. J Neurochem 71(1):288–294
Nishimura K, Shiina R, Kashiwagi K, Igarashi K (2006) Decrease in polyamines with aging and their ingestion from food and drink. J Biochem 139(1):81–90
Nunemaker CS, DeFazio RA, Moenter SM (2003) Calcium current subtypes in GnRH neurons. Biol Reprod 69(6):1914–1922
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33(3):290–295
Prokop JW, Bupp CP, Frisch A, Bilinovich SM, Campbell DB, Vogt D, Bachmann AS (2021) Emerging role of ODC1 in neurodevelopmental disorders and brain development. Genes 12(4):470
Radovick S (2012) Estrogenic regulation of the GnRH neuron. Front Endocrinol 3:52
Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC (1985) Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab 61(5):851–858
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630. https://doi.org/10.1126/science.1106943
Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K ... Olson L (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Nat Acad Sci 107(46):20087–20092
Ryu BJ, Kim HR, Jeong JK, Lee BJ (2011) Regulation of the female rat estrous cycle by a neural cell-specific epidermal growth factor-like repeat domain containing protein, NELL2. Mol Cells 32(2):203–207
Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S (2021) Polyamines: functions, metabolism, and role in human disease management. Med Sci 9(2):44
Sarchielli E, Comeglio P, Squecco R, Ballerini L, Mello T, Guarnieri G, Morelli A (2017) Tumor necrosis factor-α impairs kisspeptin signaling in human gonadotropin-releasing hormone primary neurons. J Clin Endocrinol Metab 102(1):46–56
Scarbrough K, Wise PM (1990) Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology 126(2):884–890
Schroeder S, Hofer SJ, Zimmermann A, Pechlaner R, Dammbrueck C, Pendl T, Madeo F (2021) Dietary spermidine improves cognitive function. Cell Rep 35(2):108985
Shrestha PK, Briski KP (2015) Hindbrain lactate regulates preoptic gonadotropin-releasing hormone (GnRH) neuron GnRH-I protein but not AMPK responses to hypoglycemia in the steroid-primed ovariectomized female rat. Neuroscience 298:467–474
Smith JT, Ross Young I, Veldhuis JD, Clarke IJ (2012) Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system. Endocrinology 153(7):3368–3375
Sonenberg N, Hinnebusch AG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136(4):731–745
Stevenson EL, Corella KM, Chung WC (2013) Ontogenesis of gonadotropin-releasing hormone neurons: a model for hypothalamic neuroendocrine cell development. Front Endocrinol 4:89
Taksande BG, Kotagale NR, Nakhate KT, Mali PD, Kokare DM, Hirani K, Ugale RR (2011) Agmatine in the hypothalamic paraventricular nucleus stimulates feeding in rats: involvement of neuropeptide Y. Br J Pharmacol 164(2b):704–718
Taksande BG, Sharma O, Aglawe MM, Kale MB, Gawande DY, Umekar MJ, Kotagale NR (2017) Acute orexigenic effect of agmatine involves interaction between central α2-adrenergic and GABAergic receptors. Biomed Pharmacother 93:939–947
Tao Y, Liu XJ (2013) Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell 12(1):42–49
Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, Baselga J (2017) PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355(6331):1324–1330
Tsutsui K, Ubuka T (2020) Discovery of gonadotropin-inhibitory hormone (GnIH), progress in GnIH research on reproductive physiology and behavior and perspective of GnIH research on neuroendocrine regulation of reproduction. Mol Cell Endocrinol 514:110914
Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Sharp PJ (2000) A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 275(2):661–667
Tsutsui K, Son YL, Kiyohara M, Miyata I (2018) Discovery of GnIH and its role in hypothyroidism-induced delayed puberty. Endocrinology 159(1):62–68
Tsutsumi R, Webster NJ (2009) GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J 56(6):729–737
Uzbay TI (2012) The pharmacological importance of agmatine in the brain. Neurosci Biobehav Rev 36(1):502–519
Wang X, Levic S, Gratton MA, Doyle KJ, Yamoah EN, Pegg AE (2009) Spermine synthase deficiency leads to deafness and a profound sensitivity to α-difluoromethylornithine*♦. J Biol Chem 284(2):930–937
Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, Bazer FW (2014) Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90(4):84–91
Yin W, Gore AC (2006) Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction 131(3):403–414
Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK (2009) 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29(34):10552–10562
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Cai D (2013) Hypothalamic programming of systemic aging involving IKK-β, NF-Κb and GnRH. Nature 497(7448):211–216
Zhu MY, Wang WP, Bissette G (2006) Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience 141(4):2019–2027
Acknowledgements
Work was supported by Science and Engineering Research Board (EMR/2017/004290) and Department of Biotechnology, Ministry of Science and Technology (BT/PR32910/MED/97/473/2020), New Delhi, India, financial assistance provided to Dr. Arnab Banerjee. SERB-NPDF (PDF/2017/000989) Moitreyi Das. Financial assistance from UGC-CSIR JRF provided to Nayan Anand Mate. We also thank Dr. G. Karthikeyan, BITS Pilani KK Birla Goa Campus, Goa, India, for providing the microscope facility. And we thank Dr Kazuyoshi Tsutsui Laboratory of Integrative Brain Sciences, Department of Biology, Center for Medical Life Science of Waseda University, Waseda University, Tokyo, Japan for the generous gift of GnIH antibody.
Author information
Authors and Affiliations
Contributions
Dr. AB conceived and designed the study, drafted and approved of the manuscript. NAM designed and performed major experiments and analyzed the data. MD conducted some experiments. NAM and Dr. AB wrote the first draft of the manuscript. SJ carried out the quantification of protein expression by the metric of relative pixel count method. RS analyzed RNA seq data and also contributed in writing the manuscript. All authors read, edited, reassessed the studies and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Handling editor: S. Beninati.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mate, N., Shaji, R., Das, M. et al. Expression of polyamines and its association with GnRH-I in the hypothalamus during aging in rodent model. Amino Acids 54, 1135–1154 (2022). https://doi.org/10.1007/s00726-022-03139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03139-3