Abstract
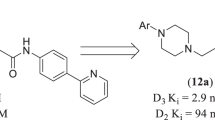
Dopamine is an important neurotransmitter that regulates numerous essential functions, including cognition and voluntary movement. As such, it serves as an important scaffold for synthesis of novel analogues as part of drug development effort to obtain drugs for treatment of neurodegenerative diseases, such as Parkinson's disease. To that end, similarity search of the ZINC database based on two known dopamine-1 receptor (D1R) agonists, dihydrexidine (DHX) and SKF 38393, respectively, was used to predict novel chemical entities with potential binding to D1R. Three compounds that showed the highest similarity index were selected for synthesis and bioactivity profiling. All main synthesis products as well as the isolated intermediates, were properly characterized. The physico-chemical analyses were performed using HRESIMS, GC/MS, LC/MS with UV–Vis detection, and FTIR, 1H NMR and 13C NMR spectroscopy. Binding to D1 and D2 receptors and inhibition of dopamine reuptake via dopamine transporter were measured for the synthesized analogues of DHX and SKF 38393.








Similar content being viewed by others
References
Arustamyan et al (1979) Arm Khim Zh 32(9):739–743
Beaulieu J-M, Espinoza S, Gainetdinov RR (2015) Dopamine receptors—IUPHAR review 13. Br J Pharmacol 172(1):1–23. https://doi.org/10.1111/bph.12906
Bemis JC, Seegal RF (2004) PCB-Induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci 80(2):288–295. https://doi.org/10.1093/toxsci/kfh153
Björklund A, Dunnett SB (2007) Fifty years of dopamine research. Trends Neurosci 30(5):185–187. https://doi.org/10.1016/j.tins.2007.03.004
Brandão ML, Coimbra NC (2019) Understanding the role of dopamine in conditioned and unconditioned fear. Rev Neurosci 30(3):325–337. https://doi.org/10.1515/revneuro-2018-0023
Cadet JL, Jayanthi S, McCoy MT et al (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets 9:526–538. https://doi.org/10.2174/187152710793361496
Carrión OA, Stamelou M, Murillo-Rodríguez E et al (2010) Dopaminergic reward system: a short integrative review. Int Arch Med. https://doi.org/10.1186/1755-7682-3-24
Chien EYT, Liu W, Zhao Q et al (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330(6007):1091–1095. https://doi.org/10.1126/science.1197410
Cisneros C, Bautista T, Betancourt CF et al (2021) Formic acid ionization and fragmentation by multiphoton absorption. J Nucl Phys Mater Sci Radiat Appl 8(2):197–201. https://doi.org/10.15415/jnp.2021.82026
Clarke HT, Gillespie HB, Weisshaus SZ (1933) The action of formaldehyde on amines and amino acids. J Am Chem Soc 55(11):4571–4587
Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69(12):e113–e125. https://doi.org/10.1016/j.biopsych.2011.03.028
Crooks PA, Szyndler R, Cox B (1980) 5,6-and 6,7-Dihydroxyspiro (tetralin-1,3´-pyrrolidine): conformationally restricted analogues of dopamine. Pharm Acta Helv 55(5):134–137
Davoren JE, Nason D, Coe J et al (2018) Discovery and lead optimization of atropisomer D1 agonists with reduced desensitization. J Med Chem 61(24):11384–11397. https://doi.org/10.1021/acs.jmedchem.8b01622
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) A rapid method for quantifying heavy atom derivatives for multiple isomorphous replacement in protein crystallography. J Appl Crystallogr 42(2):339–341
Fan L, Tan L, Chen Z et al (2020) Haloperidol bound D2 dopamine receptor structure inspired the discovery of subtype selective ligands. Nature. https://www.nature.com/articles/s41467-020-14884-y. Accessed 27 Apr 2021.
Fu H-C, You F, Li H-R, He L-N (2019) CO2 capture and in situ catalytic transformation. Front Chem 7:525. https://doi.org/10.3389/fchem.2019.00525
George MS, Molnar CE, Grenesko EL et al (2007) A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D1 agonist, is safe and tolerated in patients with schizophrenia. Schizophr Res 93(1–3):42–50. https://doi.org/10.1016/j.schres.2007.03.011
Goddard JD, Yamaguchi Y, Schaefer HF (1992) The decarboxylation and dehydration reactions of monomeric formic acid. J Chem Phys 96(2):1158–1166. https://doi.org/10.1063/1.462203
Hersch SM, Ciliax BJ, Gutekunst CA et al (1995) Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neuroscience 15:5222–5237
Hübschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44(6):1281–1284. https://doi.org/10.1107/S0021889811043202
Im D, Inoue A, Fujiwara T et al (2020) Structure of the dopamine D2 receptor in complex with the antipsychotic drug spiperone. Nat Commun 11(1):1–11. https://doi.org/10.1038/s41467-020-20221-0
Johnson F, Nasutavicus WA (1964) Polyfunctional aliphatic compounds. V. The cyclization of dinitriles by halogen acids. A new synthesis of imidazoles. J Org Chem 29(1):153–157
Kapur S, Mamo D (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27(7):1081–1090. https://doi.org/10.1016/j.pnpbp.2003.09.004
Krapcho AP, Weimaster JF (1980) Stereochemistry of decarbalkoxylation of cyclic geminal diesters effected by water and lithium chloride in dimethyl sulfoxide. J Org Chem 45(21):4105–4111
Kristensen AS, Andersen J, Jorgensen TN et al (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63(3):585–640. https://doi.org/10.1124/pr.108.000869
Machara A, Hudlický T (2016) Advances in N-and O-demethylation of opiates. Targets Heterocycl Syst 20:113–138
Mao Q, Qin W-Z, Zhang A, Ye N (2020) Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol Sin 41(4):471–482. https://doi.org/10.1038/s41401-020-0365-y
Markaryan et al. (1972) Sintezy Geterotsiklicheskikh Soedinenil 9:39–40
Marsden CA (2006) Dopamine: the rewarding years. Br J Pharmacol 147(S1):S136–S144. https://doi.org/10.1038/sj.bjp.0706473
Martel JC, McArthur SG (2020) Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol 11:1003. https://doi.org/10.3389/fphar.2020.01003
Micovic VM, Mihailovic MLJ (1953) The reduction of acid amides with lithium aluminum hydride. J Org Chem 18(9):1190–1200
Mishra A, Singh S, Shukla S (2018) Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci 12:1–8. https://doi.org/10.1177/1179069518779829
Money KM, Stanwood GD (2013) Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 7:260. https://doi.org/10.3389/fncel.2013.00260
Monte-Silva K, Kuo MF, Thirugnanasambandam N et al (2009) Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci 29(19):6124–6131. https://doi.org/10.1523/JNEUROSCI.0728-09.2009
Moraes MA, Árabe LB, Resende BL et al (2021) Effects of L-Dopa, SKF-38393 and Quinpirole on exploratory, anxiety- and depressive-like behaviors in pubertal female and male mice. bioRxiv. https://doi.org/10.1101/2021.02.26.433029
Mullard A (2017) 2016 FDA drug approvals. Nat Rev Drug Discov 16(2):73–76. https://doi.org/10.1038/nrd.2017.14
Mullard A (2018) 2017 FDA drug approvals. Nat Rev Drug Discov 17(2):81–85. https://doi.org/10.1038/nrd.2018.4
Mullard A (2019) 2018 FDA drug approvals. Nat Rev Drug Discov 18(2):85–89. https://doi.org/10.1038/d41573-019-00014-x
Mullard A (2020) 2019 FDA drug approvals. Nat Rev Drug Discov 19(2):79–84. https://doi.org/10.1038/d41573-020-00001-7
Mullard A (2021) 2020 FDA drug approvals. Nat Rev Drug Discov 20(2):85–90. https://doi.org/10.1038/d41573-021-00002-0
Olguín HJ, Guzmán DC, García EH, Mejía GB (2016) The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. https://doi.org/10.1155/2016/9730467
Osby JO, Heinzman SW, Ganem B (1986) Studies on the mechanism of transition-metal-assisted sodium borohydride and lithium aluminum hydride reductions. J Am Chem Soc 108(1):67–72
Pereira FS, deAzevedo ER, Silva EF et al (2008) Study of the carbon dioxide chemical fixation-activation by guanidines. Tetrahedron 64(43):10097–10106. https://doi.org/10.1016/j.tet.2008.08.008
Pereira FS, Agostini DLS, Santo RDE et al (2011) A comparative solid state 13C NMR and thermal study of CO2 capture by amidines PMDBD and DBN. Green Chem 13(8):2146–2153. https://doi.org/10.1039/c1gc15457e
Pérez ER, Silva MO, Costa VC et al (2002) Efficient and clean synthesis of N-alkyl carbamates by transcarboxylation and O-alkylation coupled reactions using a DBU–CO2 zwitterionic carbamic complex in aprotic polar media. Tetrahedron Lett 43(22):4091–4093. https://doi.org/10.1016/S0040-4039(02)00697-4
Pérez ER, Santos RHA, Gambardella MTP et al (2004) Activation of carbon dioxide by bicyclic amidines. J Org Chem 69(23):8005–8011. https://doi.org/10.1021/jo049243q
Pifl C, Hornykiewicz O et al (1996) Catecholamine transporters and 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity: studies comparing the cloned human noradrenaline and human dopamine transporter. J Pharmacol Exp Ther 277(3):1437–1443
Pifl C, Reither H, Hornykiewicz O (2015) The profile of mephedrone on human monoamine transporters differs from 3, 4-methylenedioxymethamphetamine primarily by lower potency at the vesicular monoamine transporter. Eur J Pharmacol 755:119–126. https://doi.org/10.1016/j.ejphar.2015.03.004
Rosell DR, Zaluda LC, McClure MM et al (2015) Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology 40(2):446–453. https://doi.org/10.1038/npp.2014.192
Savourey S, Lefèvre G, Berthet JC, Cantat T (2014) Catalytic methylation of aromatic amines with formic acid as the unique carbon and hydrogen source. Chem Commun 50(90):14033–14036. https://doi.org/10.1039/C4CC05908E
Shang J, Liu S, Ma X et al (2012) A new route of CO2 catalytic activation: syntheses of N-substituted carbamates from dialkyl carbonates and polyureas. Green Chem 14(10):2899–2906. https://doi.org/10.1039/c2gc36043h
Sheldrick GM (1996) Program for empirical absorption correction of area detector data. University of Göttingen, Göttingen
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64(1):112–122. https://doi.org/10.1107/S0108767307043930
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71(1):3–8. https://doi.org/10.1107/S2053229614024218
Slifstein M, Suckow RF, Javitch JA et al (2011) Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab 31(1):293–304. https://doi.org/10.1038/jcbfm.2010.91
Spek AL (2009) Structure validation in chemical crystallography. Acta Crystallogr D Biol Crystallogr 65(2):148–155. https://doi.org/10.1107/S090744490804362X
Stöckigt J, Antonchick AP, Wu F, Waldmann H (2011) The Pictet-Spengler reaction in nature and in organic chemistry. Angew Chem Int Ed 50(37):8538–8564. https://doi.org/10.1002/anie.201008071
Trincado M, Grützmacher H, Prechtl MHG (2018) CO2-based hydrogen storage—hydrogen generation from formaldehyde/water. Phys Sci Rev 3(5):20170013. https://doi.org/10.1515/psr-2017-0013
Waldhoer M, Cardona EB, Miligan G et al (1998) Differential uncoupling of A1 adenosine and D2 dopamine receptors by suramin and didemethylated suramin (NF037). Mol Pharmacol 53(5):808–818
Wang H, Shao Y, Zheng H et al (2015) Cyanoacetic acid as a masked electrophile: transition-metal-free cyanomethylation of amines and carboxylic acids. Chem Eur J 21(50):18333–18337. https://doi.org/10.1002/chem.201502733
Wang S, Wacker D, Levit A et al (2017) D4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science 358(6361):381–386. https://doi.org/10.1126/science.aan5468
Wang S, Che T, Levit A et al (2018) Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 555(7695):269–273. https://doi.org/10.1038/nature25758
Wu B, Zhang J, Yang M et al (2008) Raney Ni/KBH4: an efficient and mild system for the reduction of nitriles to amines. ARKIVOC 12:95–102
Xiao P, Yan W, Gou L et al (2021) Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell 184(4):943–956. https://doi.org/10.1016/j.cell.2021.01.028
Yeragani VK, Tancer M, Chokka P, Baker GB (2010) Arvid Carlsson, and the story of dopamine. Indian J Psychiatry 52(1):87
Yin J, Chen KYM, Clark MJ et al (2021) Structure of a D2 dopamine receptor–G-protein complex in a lipid membrane. Nature 584(7819):125–129. https://doi.org/10.1038/s41586-020-2379-5
Acknowledgements
The authors acknowledge for support the Centre for X-ray Structure Analysis and the Mass Spectrometry Centre of the Faculty of Chemistry, University of Vienna. Eduardo R. Perez Gonzalez is grateful to Foundation for Research Support of São Paulo State (FAPESP) for an international research fellowship (2016/10149-0) and research financial support (2018/00581-7). Suzane Rosa da Silva thanks Council for Research Support (CAPES) and Postgraduation program for Science and Technology of Materials (POSMAT) for a MSc fellowship. Luana Ribeiro dos Anjos thanks to PROPE-Unesp and National Council for Scientific and Technological Development (CNPq) for undergraduate research fellowship. Authors from LQOF thank to IEAMAR-MCTI-Unesp for 500 MHz Bruker NMR apparatus.
Funding
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential interest conflict.
Human/animals rights
The work involves no human or animals.
Informed consent
Authors consent to publish all the information in the manuscript.
Additional information
Handling editor: R. Hasmukhlal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, S.R., Kalaba, P., Fabišiková, A. et al. Synthesis and dopamine receptor binding of dihydrexidine and SKF 38393 catecholamine-based analogues. Amino Acids 54, 85–98 (2022). https://doi.org/10.1007/s00726-021-03106-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-03106-4