Abstract
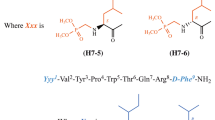
In the present study, several new analogues of hemorphin-4, modified with unnatural conformationally restricted amino acids followed the structure Aaa–Tyr–Xxx–Trp–Thr–NH2, where Aaa is the low-molecular-weight lipophilic adamantyl building block, and Xxx is Ac5c (1-aminocyclopentanecarboxylic acid) or Ac6c (1-aminocyclohexane carboxylic acid) was synthesized, characterized and investigated for anticonvulsant activity in three seizure tests, the maximal electroshock test (MES), 6-Hz psychomotor seizure test and timed intravenous pentylenetetrazole infusion (ivPTZ) test. The acute neurological toxicity was determined using the rota-rod test. The new synthetic neuropeptide analogues were prepared by solid-phase peptide synthesis—Fmoc chemistry and were evaluated in three doses of 1, 3 and 5 µg, respectively, administered intracerebroventricularly in male ICR mice. The physicochemical properties of these peptide analogues were evaluated as pKa and pI values were calculated using potentiometry. The IR spectrum of the compounds was recorded and the characteristic lines of both adamantane moiety and the peptide backbone were registered in the wavelength range from 4000 to 400 cm−1. The hexapeptide Ang IV was used as a positive control. From the six synthesized peptide analogues, the P4-5 was the most active at doses of 1 and 3 µg in the three seizure tests. The order of potency of other peptides was as follows: P4 > P4-3 = P4-4 > P4-2 > Ang IV in MES, P4-4 ≥ P4-1 > P4-3 > P4-2 > P4 > Ang IV in 6-Hz test and P4-4 = P4-3 > P4-2 = P4 > Ang IV in ivPTZ test. None of the peptides displayed neurotoxicity in the rota-rod test. Docking study results suggest that direct H-bonding and ionic interactions between our synthetic ligands and residues, responsible for coordination of Zn2+ along with hydrophobic interactions between our ligands and IRAP active site are the most important for the ligand binding. The results propose that incorporation of adamantane and cycloalkane building blocks in the peptide chain of the hemorphin-4 scaffold is important for the potential high biological activity.










Similar content being viewed by others
References
Adochitei A, Dochioiu G (2011) Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev Roum Chim 56:783–791
Alachkar A, Ojha SK, Sadeq A, Adem A, Frank A, Stark H, Sadek B (2020) Experimental models for the discovery of novel anticonvulsant drugs: focus on pentylenetetrazole-induced seizures and associated memory deficits. Curr Pharm Des 26:1693–1711
Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM, Chai SY (2001) Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. J Biol Chem 276:48263–48266
Albiston AL, Pham V, Ye S, Ng L, Lew RA, Thompson PE, Holien JK, Morton CJ, Parker MW, Chai SY (2010) Phenylalanine-544 plays a key role in substrate and inhibitor binding by providing a hydrophobic packing point at the active site of insulin-regulated aminopeptidase. Mol Pharmacol 78:600–607
Ali A, Baby B, Soman SS, Vijayan R (2019) Molecular insights into the interaction of hemorphin and its targets. Sci Rep 9:14747
Ali A, Alzeyoudi SAR, Almutawa SA, Alnajjar AN, Vijayan R (2020) Molecular basis of the therapeutic properties of hemorphins. Pharmacol Res 158:104855
Andersson H, Hallberg M (2012) Discovery of inhibitors of insulin-regulated aminopeptidase as cognitive enhancers. Int J Hypertens 2012:789671
Barlow N, Vanga SR, Sävmarker J, Sandström A, Burns P, Hallberg A, Åqvist J, Gutiérrez-De-Terán H, Hallberg M, Larhed M, Chai SY, Thompson PE (2020) Macrocyclic peptidomimetics as inhibitors of insulin-regulated aminopeptidase (IRAP). RSC Med Chem 11:234–244
Bistričić L, Baranović G, Mlinarić-Majerski K (1995) A vibrational assignment of adamantane and some of its isotopomers. Empirical versus scaled semiempirical force field. Spectrochim Acta A 51:1643
Blishchenko EY, Sazonova OV, Kalinina OA, Yatskin ON, Philippova MM, Surovoy AY, Karelin AA, Ivanov VT (2002) Family of hemorphins: co-relations between amino acid sequences and effects in cell cultures. Peptides 23:903–910
Brantl V, Gramsch C, Lottspeich F, Mertz R, Jaeger KH, Herz A (1986) Novel opioid peptides derived from hemoglobin: hemorphins. Eur J Pharmacol 125:309–310
Bryans JS, Davies N, Gee NS, Dissanayake VUK, Ratcliffe GS, Horwell DC, Knee CO, Morrell AI, Oles RJ, O’Toole JC, Perkins GM, Singh L (1998) Identification of novel ligands for the gabapentin binding site on the α2δ subunit of a calcium channel and their evaluation as anticonvulsant agents. J Med Chem 41:1838–1845
Champion HC, Zadina JE, Kastin AJ, Hackler L, Ge LJ, Kadowitz PJ (1997) Endomorphin 1 and 2, endogenous ligands for the m-opioid receptor, decrease cardiac output, and total peripheral resistance in the rat. Peptides 18:1393–1397
Chang KJ, Killian A, Hazum E, Cuatrecasas P, Chang JK (1981) Morphiceptin (NH4–Tyr–Pro–Phe–Pro–CONH2): a potent and specific agonist for morphine (µ) receptors. Science 212:75–77
Chang KJ, Cuatrecasas P, Wei ET, Chang JK (1982) Analgesic activity of intracerebroventricular administration of morphiceptin and b-casomorphins: correlation with the morphine (µ) receptor binding affinity. Life Sci 30:1547–1551
Chang KJ, Wei ET, Killian A, Chang JK (1983) Potent morphi-ceptin analogs: structure activity relationships and morphine-like activities. J Pharmacol Exp Ther 227:403–408
Chebib M, Johnston GAR (2000) GABA-activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem 43:1427
Chiba T, Li YH, Yamane T, Ogikubo O, Fukuoka M, Arai R, Takahashi S, Ohtsuka T, Ohkubo I, Matsui N (2003) Inhibition of recombinant dipeptidyl peptidase III by synthetic hemorphin-like peptides. Peptides 24:773–778
Cohen M, Fruitier-Arnaudin I, Piot JM (2004) Hemorphins: substrates and/or inhibitors of dipeptidyl peptidase IV hemorphins N-terminus sequence influence on the interaction between hemorphins and DPPIV. Biochimie 86:31–37
Czapla MA, Champion HC, Zadina JE, Kastin AJ, Hackler L, Ge LJ, Kadowitz PJ (1998) Endomorphins 1 and 2, endogenous m-opioid agonists, decrease systemic arterial pressure in the rat. Life Sci 62:175–179
Diwakarla S, Nylander E, Grönbladh A et al (2016) Binding to and inhibition of insulin-regulated aminopeptidase by macrocyclic disulfides enhances spine density. Mol Pharmacol 89:413–424
Erchegyi J, Kastin AJ, Zadina JE, Qiu XD (1992) Isolation of a heptapeptide Val–Val–Tyr–Pro–Trp–Thr–Gln (valorphin) with some opiate activity. Int J Pept Prot Res 39:477–484
Field MJ, Li Z, Schwarz JB (2007) Ca2+ channel α2-δ ligands for the treatment of neuropathic pain. J Med Chem 50:2569–2575
Fruitier I, Garreau I, Piot JM (1998) Cathepsin D is a good candidate for the specific release of a stable hemorphin from hemoglobin in vivo: VV-hemorphin-7. Biochem Biophys Res Commun 246:719–724
Fülöp F (2001) The chemistry of 2-aminocycloalkanecarboxylic acids. Chem Rev 101:2181–2204
Gademann K, Hintermann T, Scheiber JV (1999) Beta-peptides: twisting and turning. Curr Med Chem 6:905–925
Garreau I, Zhao Q, Pejoan C, Cupo A, Piot J (1995) VV-hemorphin-7 and LVV-hemorphin-7 released during in vitro peptic hemoglobin hydrolysis are morphinomimetic peptides. Neuropeptides 28:243–250
Glämsta EL, Marklund A, Hellman U, Wernstedt CH, Terenius L, Nyberg F (1991) Isolation and characterization of hemoglobin derived opioid peptides from the human pituitary gland. Regul Pept 34:169–179
Glämsta EL, Meyerson B, Silberring J, Terenius L, Nyberg F (1992) Isolation of a hemoglobin-derived opioid peptide from cerebrospinal fluid of patients with cerebrovascular bleedings. Biochem Biophys Res Commun 184:1060–1066
Gredicak M, Supek F, Kralj M, Majer Z, Hollosi M, Smuc T, Mlinaric-Majerski K, Horvat S (2010) Computational structure–activity study directs synthesis of novel antitumor enkephalin analogs. Amino Acids 38:1185–1191
Hajfathalian M, Ghelichi S, García Moreno PJ, Sørensen ADM, Jacobsen C (2018) Peptides: production, bioactivity, functionality, and applications. Crit Rev Food Sci Nutr 58:3097–3129
Hermans SJ, Ascher DB, Hancock NC, Holien JK, Michell BJ, Chai SY, Morton CJ, Parker MW (2015) Crystal structure of human insulin-regulated aminopeptidase with specificity for cyclic peptides. Protein Sci 24:190–199
Jinsmaa Y, Yoshikawa M (2002) Release of hemorphin-5 from human hemoglobin by pancreatic elastase. Biosci Biotechnol Biochem 66:1130–1132
Johnston GAR (2005) GABAA receptor channel pharmacology. Curr Pharm Design 11:1867–1885
Kaiser E, Colescott RL, Bossinger CD, Cook PI (1970) Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem 34:595–598
Kozlowski LP (2016) IPC—isoelectric point calculator. Biol Direct 11:55
Krall R, Penry J, White B, Kupferberg H, Swinyard E (1978) Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 19:409–428
Krimm S, Bandekar J (1986) Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem 38:181–364
Krogsgaard-Larsen P, Frolund B, Frydenvang K (2000) GABA uptake inhibitors. design, molecular pharmacology and therapeutic aspects. Curr Pharm Design 6:1193–1209
Lee J, Mustafa T, Mcdowall SG, Mendelsohn FAO, Brennan M, Lew RA, Albiston AL, Chai SY (2003a) Structure–activity study of LVV-hemorphin-7: angiotensin AT4 receptor ligand and inhibitor of insulin-regulated aminopeptidase. J Pharmacol Exp Ther 305:205–211
Lee YC, Zocharski PD, Samas B (2003b) An intravenous formulation decision tree for discovery compound formulation development. Int J Pharm 253:111–119
Liebmann C, Schrader U, Brantl V (1989) Opioid receptor affinities of the blood-derived tetrapeptides hemorphin and cytochrophin. Eur J Pharmacol 166:523–526
Liu J, Obando D, Liao V, Lifa T, Codd R (2011) The many faces of the adamantyl group in drug design. Eur J Med Chem 46:1949–1963
Moeller I, Lew RA, Mendelsohn FA, Smith AI, Brennan ME, Tetaz TJ, Chai SY (1997) The globin fragment LVV-hemorphin-7 is an endogenous ligand for the AT4 receptor in the brain. J Neurochem 68:2530–2537
Montecucchi PC, de Castiglione R, Erspamer V (1981) Identification of dermorphin and Hyp6-dermorphin in skin extracts of the Brazilian frog Phyllomedusa rhodei. Int J Pept Protein Res 17:316–321
Mortensen UH, Raaschou-Nielsen M, Breddam K (1994) Recognition of C-terminal amide groups by (serine) carboxypeptidase Y investigated by site-directed mutagenesis. J Biol Chem 269:15528
Nema S, Ludwig JD (2019) Parenteral medications. CRC Press, Boca Raton, pp 193–194 (ISBN: 0429576838, 9780429576836)
Nutt DJ, Taylor SC, Little HJ (1986) Optimizing the pentetrazol infusion test for seizure threshold measurements. J Pharm Pharmacol 38:697–698
Nyberg F, Sanderson K, Glämsta EL (1997) The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 43:147–156
Ojima I, Lin S, Wang T (1999) Recent advances in the medicinal chemistry of taxoids with novel beta-amino acid side chains. Curr Med Chem 6:927–954
Pogozheva ID, Przydzial MJ, Mosberg HI (2005) Homology modeling of opioid receptor-ligand complexes using experimental constraints. The AAPS Journal 7:434–448
Prasad S, Rao RB, Balaram P (1995) Contrasting solution conformations of peptides containing α, α-dialkylated residues with linear and cyclic side chains. Biopolymers 35:11–20
Sarin VK, Kent SBH, Tam JP, Merrifield RB (1986) Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal Biochem 117:147–157
Shundalau M, Mindarava YL, Matsukovich AS, Gaponenko SV, El-Emam AA, Alkahtani HN (2019) Structural, Vibrational and UV/Vis Studies of Adamantane-Containing Triazole Thiones by Spectral, DFT and Multi-reference ab initio Methods. Z Phys Chem 234:85–106
Stevenazzi A, Marchini M, Sandrone G, Vergani B, Lattanzio M (2014) Amino acidic scaffolds bearing unnatural side chains: An old idea generates new and versatile tools for the life sciences. Bioorg Med Chem Lett 24:5349–5356
Tchekalarova J, Angelova V, Todorova N, Andreeva-Gateva P, Rangelov M (2019) Evaluation of the anticonvulsant effect of novel melatonin derivatives in the intravenous pentylenetetrazol seizure test in mice. Eur J Pharmacol 863:172684
Todorov P, Peneva P, Pechlivanova D, Georgieva S, Dzhambazova E (2018) Synthesis, characterization and nociceptive screening of new VV-hemorphin-5 analogues. Bioorg Med Chem Lett 28:3073–3079
Todorov P, Peneva P, Georgieva S, Tchekalarova J, Vitkova V, Antonova K, Georgiev A (2019a) Synthesis, characterization and anticonvulsant activity of new azobenzene-containing VV-hemorphin-5 bio photoswitch. Amino Acids 51:549–563
Todorov P, Peneva P, Tchekalarova J, Rangelov M, Georgieva S, Todorova N (2019b) Synthesis, characterization and anticonvulsant activity of new series of N-modified analogues of VV-hemorphin-5 with aminophosphonate moiety. Amino Acids 51:1527–1545
Todorov P, Rangelov M, Peneva P, Todorova N, Tchekalarova J (2019c) Anticonvulsant evaluation and docking analysis of VV-Hemorphin-5 analogues. Drug Dev Res 80:425–437
Todorov P, Peneva P, Tchekalarova J, Georgieva S (2020) Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: synthesis and characterization. Amino Acids 52:567–585
Wanka L, Iqbal K, Schreiner PR (2013) The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem Rev 113:3516–3604
Watkins AM, Craven TW, Renfrew PD, Arora PS, Bonneau R (2017) Rotamer Libraries for the High-Resolution Design of β-Amino Acid Foldamers. Structure 25:1771–1780
Yang YR, Chiu TH, Chen CL (1999) Structure–activity relationships of naturally occurring and synthetic opioid tetrapeptides acting on locus coeruleus neurons. Eur J Pharmacol 372:229–236
Ye S, Chai SY, Lew RA, Albiston AL (2007) Insulin-regulated aminopeptidase: analysis of peptide substrate and inhibitor binding to the catalytic domain. Biol Chem 388:399–403
Zadina JE, Paul D, Gergen KA, Ge LJ, Hackler L, Kastin AJ (1996) Binding of Tyr-W-MIF-1 (Tyr–Pro–Trp–Gly–NH2) and related peptides to m1 and m2 opiate receptors. Neurosci Lett 215:65–69
Zadina JE, Hackler L, Ge LJ, Kastin AJ (1997) A potent and selective endogenous agonist for the µ-opiate receptor. Nature 386:499–502
Acknowledgements
This work was financially supported by the Bulgarian National Scientific Fund project КП-06-OПP 03/3 of the Ministry of Education and Science, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures were performed in agreement with the European Communities Council Directive 2010/63/EU. The experimental design was approved by the Institutional Ethics Committee. There are no human participants.
Additional information
Handling Editor: F. Albericio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Todorov, P., Peneva, P., Tchekalarova, J. et al. Structure–activity relationship study on new hemorphin-4 analogues containing steric restricted amino acids moiety for evaluation of their anticonvulsant activity. Amino Acids 52, 1375–1390 (2020). https://doi.org/10.1007/s00726-020-02898-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-020-02898-1