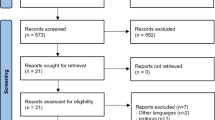
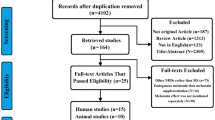
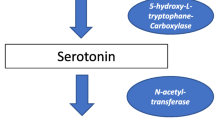
Abstract
It is well recognized that variation in the geographical distribution of prevalence of multiple sclerosis (MS) exists: increasing the latitude its prevalence increases as well, but the underlying causes of such dissimilarity still remained elusive as of today. Currently, the most accredited hypothesis is that the closer to the equator the more pronounced is the amount of sunlight which, in turn, increases the production of vitamin D. Cholecalciferol is indeed deficient in MS patients, but this factor does not explain by itself the etiopathogenesis of the disease. In the present study, to search for a pattern and provide a model of the disease’s etiology consistent with this regional factor, as well with its changing ethnic, sex-ratio, lifestyle variations and the other unexplained aspects of MS, an extensive analysis of peer-reviewed literature and data was conducted. The arisen hypothesis was that, increasing the latitude, the factor that varies and can have the stronger effect on the human organism, is the continuous and ever-increasing diversity of the natural light–dark cycle. The consequent effort of the suprachiasmatic nucleus to entrain the organism’s circadian rhythm affects the hypothalamic–pituitary–adrenal axis resulting in desynchronizing the central and peripheral circadian clocks and pathologizing the immunitary system. To verify such hypothesis, a theoretical framework of the etiopathogenesis, coherent with the gathered literature, was conceived and a demonstration to corroborate it was eventually devised and performed. The results underscored that people living in countries subjected to a further circadian disruptive factor, as daylight saving time, have a 6.35 times higher prevalence of MS than States placed on their same latitude that do not observe it, thus strongly supporting the hypothesis. As further reinforcement of the conclusions, it is worth mentioning that the levels of polyamines rise abruptly in autoimmune diseases. Moreover, among their numerous roles, these polycations participate to the regulation of the circadian clock so their sudden variation might disrupt it. Following these interesting findings, new perspectives in therapies are, therefore, proposed.









Similar content being viewed by others
Abbreviations
- MS:
-
Multiple sclerosis
- HPA axis:
-
Hypothalamic–pituitary–adrenal axis
- DST:
-
Daylight saving time
- LD:
-
Light–dark
- SCN:
-
Suprachiasmatic nucleus
- SWS:
-
Summer winter solstice
- PD:
-
Parkinson’s disease
- NKs:
-
Natural killer
- GID:
-
Gender identity disorders
- CNS:
-
Central nervous system
- ST:
-
Standard time
References
Ali MA, Poortvliet E, Strömberg R, Yngve A (2011) Polyamines in foods: development of a food database. Food and Nutr Res 55(1):5572–5586. https://doi.org/10.3402/fnr.v55i0.5572
Alla S, Pearson J, Debernard L, Miller D, Mason D (2014) The increasing prevalence of multiple sclerosis in New Zealand. Neuroepidemiology 42(3):154–160. https://doi.org/10.1159/000358174
Anglmayer I (2017) EU summer-time arrangements under Directive 2000/84/EC. Ex-post impact assessment. (Ex-Post Evaluation unit of the directorate for impact assessment and European added value, secretariat of the European parliament. http://www.europarl.europa.eu/thinktank https://doi.org/10.2861/380995. Accessed 14 Apr 2018
Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, Schantz MV (2003) A Length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26(4):413–415. https://doi.org/10.1093/sleep/26.4.413
Ascherio A, Munger KL (2007) Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 61(4):288–299. https://doi.org/10.1002/ana.21117
Atlas of MS (2013) Mapping multiple sclerosis around the world. London: Multiple sclerosis international federation 2013. Available at: http://www.msif.org/about-ms/ publications-and-resources/. Accessed October 10, 2013
Atlas: multiple sclerosis resources in the world (2008) Geneva, Switzerland: World Health Organisation; 2008. Available at: http://www.msif.org/about-ms/publications-and-esources/. Accessed 4 Feb 2018
Battaglia MA, Bezzini D (2016) Estimated prevalence of multiple sclerosis in Italy in 2015. Neurol Sci 38(3):473–479. https://doi.org/10.1007/s10072-016-2801-9->
Beretich B, Beretich T (2009) 08) Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler J 15(8):891–898. https://doi.org/10.1177/1352458509105579
Bonmati-Carrion M, Arguelles-Prieto R, Martinez-Madrid M, Reiter R, Hardeland R, Rol M, Madrid J (2014) Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci 15(12):23448–23500. https://doi.org/10.3390/ijms151223448
Brooks WH (2013) Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Front Immunol. https://doi.org/10.3389/fimmu.2013.00091
Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ (2014) 09) Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83(11):1022–1024. https://doi.org/10.1212/wnl.0000000000000768
Cantorna M (2008) Vitamin D and multiple sclerosis: an update. Nutr Rev 66(21):S135–S138. https://doi.org/10.1111/j.1753-4887.2008.00097.x
Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ (2010) Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185(10):5796–5805. https://doi.org/10.4049/jimmunol.1001026
Chen W, Harnett MT, Smith SM (2007) Modulation of neuronal voltage-activated calcium and sodium channels by polyamines and pH. Channels 1(4):281–290. https://doi.org/10.4161/chan.498
Cree BA, Khan O, Bourdette D, Goodin DS, Cohen JA, Marrie RA, Hauser SL (2004) Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 63(11):2039–2045. https://doi.org/10.1212/01.wnl.0000145762.60562.5d
Czeisler Charles A, Duffy Jeanne F, Shanahan Theresa L, Brown Emery N, Mitchell Jude F, Rimmer David W, Ronda Joseph M, Silva Edward J, Allan James S, Emens Jonathan S, Derk-Jan Dijk, Kronauer Richard E (1999) Stability, precision, and near-24-h period of the human circadian pacemaker. Science 284(5423):2177–2181. https://doi.org/10.1126/science.284.5423.2177
Damsker JM, Hansen AM, Caspi RR (2010) Th1 and Th17 cells. Ann N Y Acad Sci 1183(1):211–221. https://doi.org/10.1111/j.1749-6632.2009.05133.x
Dean G, Kurtzke JF (1971) On the risk of multiple sclerosis according to age at immigration to south Africa. BMJ 3(5777):725–729. https://doi.org/10.1136/bmj.3.5777.725->
Dean G, Bhigjee AI, Bill PL, Fritz V, Chikanza IC, Thomas JEP, Levy LF, Saffer D (1994) Multiple sclerosis in black South Africans and Zimbabweans. J Neurol Neurosurg Psychiatry 57(9):1064–1069. https://doi.org/10.1136/jnnp.57.9.1064->
Detels R, Visscher BR, Haile RW, Malmgren RM, Dudley JP, Coulson AH (1978) Multiple sclerosis and age at migration. Am J Epidemiol 108(5):386–393. https://doi.org/10.1093/oxfordjournals.aje.a112636
Eastman CI, Molina TA, Dziepak ME, Smith MR (2012) Lacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol Int 29(8):1072–1077. https://doi.org/10.3109/07420528.2012.700670
Ebers GC, Sadovnick AD, Veith R, Munger KL, O’reilly E, Ascherio A (2004) Vitamin D intake and incidence of multiple sclerosis. Neurology 63(5):939–939. https://doi.org/10.1212/wnl.63.5.939
Edholm OG, Gunderson EKE (1973) Polar human biology. Butterworth-Heinemann, Oxford, p 313
Etchegaray J, Machida KK, Noton E, Constance CM, Dallmann R, Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, Weaver DR (2009) Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol 29(14):3853–3866. https://doi.org/10.1128/mcb.00338-09
Farez M, Mascanfroni I, Méndez-Huergo S, Yeste A, Murugaiyan G, Garo L, Correale J (2015) Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162(6):1338–1352. https://doi.org/10.1016/j.cell.2015.08.025
Field EJ (1977) Multiple sclerosis: a critical conspectus. MTP Press Limited, Lancaster, England. https://doi.org/10.1007/978-94-010-9534-1
Foster RG, Roenneberg T (2008) Human responses to the geophysical daily, annual and lunar cycles. Curr Biol. https://doi.org/10.1016/j.cub.2008.07.003
Galluzzi L, Pietrocola F, Kroemer G (2015) Molecular regulation of circadian rhythms by polyamines. Cell Metab 22(5):757–758. https://doi.org/10.1016/j.cmet.2015.10.00
Geiger SS, Fagundes CT, Siegel RM (2015) Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology 146(3):349–358. https://doi.org/10.1111/imm.12525
Ghareghani M, Sadeghi H, Zibara K, Danaei N, Azari H, Ghanbari A (2016) Melatonin increases oligodendrocyte differentiation in cultured neural stem cells. Cell Mol Neurobiol 37(7):1319–1324. https://doi.org/10.1007/s10571-016-0450-4
Gilad GM, Gilad VH (1999) Novel polyamine derivatives as neuroprotective agents. J Pharmacol Exp Ther. 291(1):39–43. http://jpet.aspetjournals.org/content/jpet/291/1/39.full.pdf. Accessed 31 May 2018
Goodin DS (2009) The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLoS One. https://doi.org/10.1371/journal.pone.0004565
Goodin DS, Cohen BA, O’connor P, Kappos L, Stevens JC (2008) Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology 71(10):766–773. https://doi.org/10.1212/01.wnl.0000320512.21919.d2
Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Reen EV, Lockley SW (2011) Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. Endocrinology 152(2):742–742. https://doi.org/10.1210/endo.152.2.zee742
Grytten N, Torkildsen Ø, Myhr K (2015) Time trends in the incidence and prevalence of multiple sclerosis in Norway during eight decades. Acta Neurol Scand 132:29–36. https://doi.org/10.1111/ane.12428
Hammond SR (2000) The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain 123(5):968–974. https://doi.org/10.1093/brain/123.5.968
Harbo HF, Gold R, Tintoré M (2013) Sex and gender issues in multiple sclerosis. Ther Adv Neurol Dis 6(4):237–248. https://doi.org/10.1177/1756285613488434->
Harrington M (2010) Location, location, location: important for jet-lagged circadian loops. J Clin Investig 120(7):2265–2267. https://doi.org/10.1172/JCI43632
Hart PH, Gorman S, Finlay-Jones JJ (2011) Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 11(9):584–596. https://doi.org/10.1038/nri3045
Hatori M, Gill S, Mure LS, Goulding M, O’leary DD, Panda S (2014) Lhx1 maintains synchrony among circadian oscillator neurons of the SCN. ELife. https://doi.org/10.7554/elife.03357
Herzog ED, Kiss IZ, Mazuski C (2015) Measuring Synchrony in the Mammalian Central Circadian Circuit. Methods Enzymol Circ Rhythms and Biol Clocks. https://doi.org/10.1016/bs.mie.2014.10.042
Hesterberg R, Cleveland J, Epling-Burnette P (2018) Role of Polyamines in Immune Cell Functions. Med Sci 6(1):22. https://doi.org/10.3390/medsci6010022->
Hood S, Amir S (2017) Neurodegeneration and the Circadian Clock. Front Aging Neurosci 9:1. https://doi.org/10.3389/fnagi.2017.00170
Huang R (2018) The discoveries of molecular mechanisms for the circadian rhythm: the 2017 Nobel Prize in Physiology or Medicine. Biomed J 41(1):5–8. https://doi.org/10.1016/j.bj.2018.02.003->
Hyppönen E, Läärä E, Reunanen A, Järvelin M, Virtanen SM (2001) Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358(9292):1500–1503. https://doi.org/10.1016/s0140-6736(01)06580-1
Ibáñez (2017) Scientific background discoveries of molecular mechanisms controlling the circadian rhythm. The Nobel Assembly at Karolinska institute, Stockholm. https://www.nobelprize.org/uploads/2018/06/advanced-medicineprize2017.pdf. Accessed 24 Feb 2018
Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M (2009) Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun 33(1):12–16. https://doi.org/10.1016/j.jaut.2009.03.005
Kantermann T, Juda M, Merrow M, Roenneberg T (2007) The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr Biol 17(22):1996–2000. https://doi.org/10.1016/j.cub.2007.10.025
Kosinski M, Wang Y, Lakkaraju H, Leskovec J (2016) Mining big data to extract patterns and predict real-life outcomes. Psychol Methods 21(4):493–506. https://doi.org/10.1037/met0000105
Krishnan HC, Lyons LC (2015) Synchrony and desynchrony in circadian clocks: impacts on learning and memory. Learn Mem 22(9):426–437. https://doi.org/10.1101/lm.038877.115c
Kurtzke JF (1983) Some epidemiological trends in multiple sclerosis. Trends Neurosci 6:75–80. https://doi.org/10.1016/0166-2236(83)90042-5->
Kurtzke JF (2000). Multiple sclerosis in time and space - Geographic clues to cause. J Neurovirol. 6(Suppl 2):S134–40. https://pdfs.semanticscholar.org/d522/df170c2f692afa82c79794e82c18399dad2a.pdf
Larcher S, Gauchez A, Lablanche S, Pépin J, Benhamou P, Borel A (2016) Impact of sleep behavior on glycemic control in type 1 diabetes: the role of social jetlag. Eur J Endocrinol 175(5):411–419. https://doi.org/10.1530/eje-16-0188
Lauretti E, Meco AD, Merali S, Praticò D (2016) Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol Psychiatry 22(2):280–286. https://doi.org/10.1038/mp.2016.47
Lavtar P, Rudolf G, Maver A, Hodžić A, Čizmarević NS, Živković M, Peterlin B (2018) Association of circadian rhythm genes ARNTL/BMAL1 and CLOCK with multiple sclerosis. PLoS One 13(1):e0190601. https://doi.org/10.1371/journal.pone.0190601
Lebailly B, Boitard C, Rogner UC (2015) Circadian rhythm-related genes: implication in autoimmunity and type 1 diabetes. Diabetes Obes Metab 17:134–138. https://doi.org/10.1111/dom.12525
Lemire JM, Archer C, Beck L, Spiegelberg HL (1995) Immunosuppressive actions of 1,25-dihydroxy vitamin D3: preferential Inhibition of Th1 Functions. J Nutr 125(6):1704S–1708S. https://doi.org/10.1093/jn/125.suppl_6.1704S
Lobban MC (1967) Daily rhythms of renal excretion in arctic-dwelling Indians and Eskimos. Q J Exp Physiol Cogn Med Sci 52(4):401–410. https://doi.org/10.1113/expphysiol.1967.sp001934
Loma I, Heyman R (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9(3):409–416. https://doi.org/10.2174/157015911796557911
Lu W, Meng Q, Tyler NJ, Stokkan K, Loudon AS (2010) A circadian clock is not required in an arctic mammal. Curr Biol 20(6):533–537. https://doi.org/10.1016/j.cub.2010.01.042
Madeo F, Eisenberg T, Pietrocola F, Kroemer G. (2018) Spermidine in health and disease. Science. 359–410(6374):eaan 2788. https://doi.org/10.1126/science.aan2788
Malekzadeh A, Geer-Peeters WV, Groot VD, Teunissen CE, Beckerman H, Group TS (2015) Fatigue in patients with multiple sclerosis: is it related to pro- and anti-inflammatory cytokines? Disease Mark 2015:1–7. https://doi.org/10.1155/2015/758314
Masters WA, McMillan MS (2001) Climate and scale in economic growth. J Econ Growth 6(3):167–186. https://doi.org/10.1023/A:101139843152
Masuko T, Kusama-Eguchi K, Sakata K, Kusama T, Chaki S, Okuyama S, Igarashi K (2003) Polyamine transport, accumulation, and release in brain. J Neurochem 84(3):610–617. https://doi.org/10.1046/j.1471-4159.2003.01558.x->
Materljan E, Sepčić J, Materljan B (2001). Sclerosi multipla e ambiente—multiple sclerosis and environment. Conference paper: ambiente e salute—environment and health, At San Marino, AIEP editore, pp 155–171
Milo R, Kahana E (2010) Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2009.11.010
Mohammed EM (2016) Multiple sclerosis is prominent in the Gulf states: review. Pathogenesis 3(2):19–38. https://doi.org/10.1016/j.pathog.2016.04.001
Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35(1):445–462. https://doi.org/10.1146/annurev-neuro-060909-153128
Mohr SB, Garland CF, Gorham ED, Garland FC (2008) The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 51(8):1391–1398. https://doi.org/10.1007/s00125-008-1061-5
Monzani F, Caraccio N, Meucci G, Lombardo F, Moscato G, Casolaro A, Ferdeghini M, Murri M, Ferrannini E (1999) Effect of 1-year treatment with interferon-beta1b on thyroid function and autoimmunity in patients with multiple sclerosis. Eur J Endocrinol 141(4):325–331. https://doi.org/10.1530/eje.0.1410325
Moretti M (2014) Role of agmatine in neurodegenerative diseases and epilepsy. Front Biosci E6(2):341. https://doi.org/10.2741/710
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxy vitamin D levels and risk of multiple sclerosis. JAMA 296(23):2832. https://doi.org/10.1001/jama.296.23.2832
Olivier P, Fontaine RH, Loron G, Steenwinckel JV, Biran V, Massonneau V, Baud O (2009) Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One. https://doi.org/10.1371/journal.pone.0007128
Pakpoor J, Wotton CJ, Schmierer K, Giovannoni G, Goldacre MJ (2016) Gender identity disorders and multiple sclerosis risk: a national record-linkage study. Mult Scler J 22(13):1759–1762. https://doi.org/10.1177/1352458515627205
Palmer AJ, Colman S, O’Leary B, Taylor BV, Simmons RD (2013) The economic impact of multiple sclerosis in Australia in 2010. Mult Scler J 19(12):1640–1646. https://doi.org/10.1177/1352458513488230
Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castano-Vinyals G, Basagana X, Ribas FC, Mirabent J, Martφn J, Carenys G, Martφn CR, Middleton B, Skene DJ, Kogevinas M (2014) Circadian variation of melatonin, light exposure, and diurnal preference in day and night shift workers of both sexes. Cancer Epidemiol Biomark Prev 23(7):1176–1186. https://doi.org/10.1158/1055-9965.epi-13-1271
Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R (2016) Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep 6(1):1. https://doi.org/10.1038/srep25970
Reifman A, Biernat M, Lang EL (1991) Stress, social support, and health in married professional women with small children. Psychol Women Q 15(3):431–445. https://doi.org/10.1111/j.1471-6402.1991.tb00419.x
Reinberg AE, Touitou Y, Soudant É, Bernard D, Bazin R, Mechkouri M (1996) Oral contraceptives alter circadian rhythm parameters of cortisol, melatonin, blood pressure, heart rate, skin blood flow, transepidermal water loss, and skin amino acids of healthy young women. Chronobiol Int 13(3):199–211. https://doi.org/10.3109/07420529609012653
Roenneberg T, Merrow M (2016) The circadian clock and human health. Curr Biol 26(10):1. https://doi.org/10.1016/j.cub.2016.04.011
Rojas-Villarraga A, Amaya-Amaya J, Rodriguez-Rodriguez A, Mantilla RD, Anaya J (2012) Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Diseases 2012:1–9. https://doi.org/10.1155/2012/254319
Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P (2012) Vitamin D as a protective factor in multiple sclerosis. Neurology 79(21):2140–2145. https://doi.org/10.1212/wnl.0b013e3182752ea8
Santhi N, Lazar AS, Mccabe PJ, Lo JC, Groeger JA, Dijk D (2016) Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1521637113
Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Franken P (2015) Clock-Talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb Symp Quant Biol 80:223–232. https://doi.org/10.1101/sqb.2015.80.027490
Selmi C, Cincinelli G, Generali E, Dudam R, Ravindran V (2018) Why women or why not men? sex and autoimmune diseases. Indian J Rheumatol 13(1):44. https://doi.org/10.4103/injr.injr_1_18->
Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, Dwyer T, Gies P, Mei IV (2010) Higher 25-hydroxyvitamin D is associated with lower relapse risk in MS. Ann Neurol. https://doi.org/10.1002/ana.22043
Skeldon AC, Phillips AJ, Dijk D (2017) The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci Rep. https://doi.org/10.1038/srep45158
Smestad C, Sandvik L, Holmoy T, Harbo HF, Celius EG (2007) Marked differences in prevalence of multiple sclerosis between ethnic groups in Oslo, Norway. J Neurol 255(1):49–55. https://doi.org/10.1007/s00415-007-0659-8
Sotgiu S, Angius A, Embry A, Rosati G, Musumeci S (2008) Hygiene hypothesis: innate immunity, malaria and multiple sclerosis. Med Hypotheses 70(4):819–825. https://doi.org/10.1016/j.mehy.2006.10.069
Sotirchos ES, Bhargava P, Eckstein C, Haren KV, Baynes M, Ntranos A, Calabresi PA (2015) Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 86(4):382–390. https://doi.org/10.1212/wnl.0000000000002316->
Sutton CE, Finlay CM, Raverdeau M, Early JO, Decourcey J, Zaslona Z, Curtis AM (2017) Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat Commun. https://doi.org/10.1038/s41467-017-02111-0
Terna SPA (2017) N 7 Mesi di ora legale minori consumi elettrici per 567 milioni di kilowattora http://download.terna.it/terna/0000/0994/35.PDF. Accessed 27 Feb 2018
United Nations (2015) Department of economic and social affairs, population division. Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349). http://www.un.org/en/development/desa/population/publications/pdf/family/trendsContraceptiveUse2015Report.pdf. Accessed 3 Mar 2018
Vijayanathan V, Agostinelli E, Thomas T, Thomas TJ (2013) Innovative approaches to the use of polyamines for DNA nanoparticle preparation for gene therapy. Amino Acids 46(3):499–509. https://doi.org/10.1007/s00726-013-1549-2
Villapol S, Fau S, Renolleau S, Biran V, Charriaut-Marlangue C, Baud O (2011) Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr Res 69(1):51–55. https://doi.org/10.1203/pdr.0b013e3181fcb40b
Wallin MT, Culpepper WJ, Coffman P, Pulaski S, Maloni H, Mahan CM, Kurtzke JF (2012) The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain 135(6):1778–1785. https://doi.org/10.1093/brain/aws099
Wehrens SM, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Johnston JD (2017) Meal timing regulates the human circadian system. Curr Biol. https://doi.org/10.1016/j.cub.2017.04.059
Wharfe MD, Mark PJ, Wyrwoll CS, Smith JT, Yap C, Clarke MW, Waddell BJ (2016) Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J Endocrinol 228(3):135–147. https://doi.org/10.1530/joe-15-0405->
White TD, Asfaw B, Degusta D, Gilbert H, Richards GD, Suwa G, Howell FC (2003) Pleistocene homo sapiens from Middle Awash, Ethiopia. Nature 423(6941):742–747. https://doi.org/10.1038/nature01669
Wu GF, Alvarez E (2011) The immunopathophysiology of multiple sclerosis. Neurol Clin 29(2):257–278. https://doi.org/10.1016/j.ncl.2010.12.009
Wulff K, Gatti S, Wettstein JG, Foster RG (2010) Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11(8):589–599. https://doi.org/10.1038/nrn2868
Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG (1998) Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension 32(3):417–423. https://doi.org/10.1161/01.hyp.32.3.417
Zahoor I, Haq E (2017) Vitamin D and multiple sclerosis: an update. In: Zagon IS, McLaughlin PJ (eds) Multiple sclerosis: perspectives in treatment and pathogenesis. Codon Publications, Brisbane. https://doi.org/10.15586/codon.multiplesclerosis.2017.ch5
Zhu M, Wang W, Cai Z, Regunathan S, Ordway G (2008) Exogenous agmatine has neuroprotective effects against restraint-induced structural changes in the rat brain. Eur J Neurosci 27(6):1320–1332. https://doi.org/10.1111/j.1460-9568.2008.06104.x
Zwighaft Z, Aviram R, Shalev M, Rousso-Noori L, Kraut-Cohen J, Golik M, Brandis A, Reinke H, Aharoni A, Kahana C, Asher G (2015) Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab 22(5):874–885. https://doi.org/10.1016/j.cmet.2015.09.011
Acknowledgements
The authors thank Anna Petrova for the helpful and fruitful suggestions and discussion as well as for the daily insights provided and Arch. Roberto Mezzaroma for having continuously encouraged the writing out of the manuscript and the drawing up of the project. Our gratitude is also due to the “International Polyamines Foundation—ONLUS” for the availability to look up in the Polyamines documentation.
Funding
This study was also supported by resources provided by SAPIENZA University of Rome, Italy (EA).
Author information
Authors and Affiliations
Contributions
FG and EA conceived this investigation and coordinated the collaboration among the authors. All the authors wrote the manuscript and all the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
The present review does not contain any studies with human participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Informed consent
For this type of study, informed consent is not required.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Handling Editor: G. Wu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gasperoni, F., Turini, P. & Agostinelli, E. A novel comprehensive paradigm for the etiopathogenesis of multiple sclerosis: therapeutic approaches and future perspectives on its treatment. Amino Acids 51, 745–759 (2019). https://doi.org/10.1007/s00726-019-02718-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02718-1