Abstract
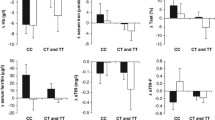
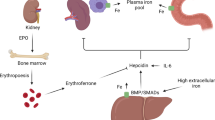
Abnormalities of iron homeostasis have been linked to insulin resistance, type 2 diabetes and cardiovascular disease. Carnosine, an over-the-counter food supplement with chelating properties, has been shown to decrease serum iron and improve glucose metabolism in diabetic rodents. We have previously demonstrated that carnosine supplementation prevented worsening of glucose metabolism in healthy overweight and obese middle-aged adults. Yet, the impact of carnosine on markers of iron metabolism in humans has not been investigated. We aimed to determine whether carnosine supplementation has an effect on iron parameters in overweight and obese, otherwise healthy adults. We included 26 participants, who were randomly allocated to receive 1 g carnosine (n = 14) or identical placebo (n = 12) twice daily for 12 weeks. Iron parameters including iron, ferritin, transferrin, soluble transferrin receptor, total iron binding capacity and iron saturation were measured in serum or plasma by standard commercial assays. Carnosine supplementation decreased plasma soluble transferrin receptor compared to placebo (mean change difference ± standard error: − 0.07 ± 0.03 mg/l, p = 0.04). None of the other iron parameters were different between carnosine and placebo groups. At follow-up, soluble transferrin receptor was associated inversely with urinary carnosine concentrations and positively with serum carnosinase-1 activity (both p < 0.02). Our findings suggest that carnosine may modulate iron metabolism in high-risk groups which could ameliorate insulin resistance and prevent type 2 diabetes. Larger human clinical trials are required to confirm our results.


Similar content being viewed by others
References
Adelmann K, Frey D, Riedl E, Koeppel H, Pfister F, Peters V, Schmitt CP, Sternik P, Hofmann S, Zentgraf HW, Navis G, van den Born J, Bakker SJ, Kramer BK, Yard BA, Hauske SJ (2012) Different conformational forms of serum carnosinase detected by a newly developed sandwich ELISA for the measurements of carnosinase concentrations. Amino Acids 43(1):143–151. https://doi.org/10.1007/s00726-012-1244-8
Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, Yeum KJ, Negrisoli G, Carini M (2011) The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med 15(6):1339–1354. https://doi.org/10.1111/j.1582-4934.2010.01101.x
Aldini G, Carini M, Yeum KJ, Vistoli G (2014) Novel molecular approaches for improving enzymatic and nonenzymatic detoxification of 4-hydroxynonenal: toward the discovery of a novel class of bioactive compounds. Free Radic Biol Med 69:145–156. https://doi.org/10.1016/j.freeradbiomed.2014.01.017
Alhamdani MS, Al-Azzawie HF, Abbas FK (2007) Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit Dial Int 27(1):86–89
Baye E, Ukropcova B, Ukropec J, Hipkiss A, Aldini G, de Courten B (2016) Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 48(5):1131–1149. https://doi.org/10.1007/s00726-016-2208-1
Baye E, Ukropec J, de Courten MPJ, Vallova S, Krumpolec P, Kurdiova T, Aldini G, Ukropcova B, de Courten B (2017) Effect of carnosine supplementation on the plasma lipidome in overweight and obese adults: a pilot randomised controlled trial. Sci Rep 7(1):17458. https://doi.org/10.1038/s41598-017-17577-7
Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93(4):1803–1845. https://doi.org/10.1152/physrev.00039.2012
Choudhuri S, Dutta D, Sen A, Chowdhury IH, Mitra B, Mondal LK, Saha A, Bhadhuri G, Bhattacharya B (2013) Role of N-epsilon- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol Vis 19:100–113
Coimbra S, Catarino C, Nascimento H, Ines Alves A, Filipa Medeiros A, Bronze-da-Rocha E, Costa E, Rocha-Pereira P, Aires L, Seabra A, Mota J, Ferreira Mansilha H, Rego C, Santos-Silva A, Belo L (2017) Physical exercise intervention at school improved hepcidin, inflammation, and iron metabolism in overweight and obese children and adolescents. Pediatr Res 82(5):781–788. https://doi.org/10.1038/pr.2017.139
Cooksey RC, Jones D, Gabrielsen S, Huang J, Simcox JA, Luo B, Soesanto Y, Rienhoff H, Abel ED, McClain DA (2010) Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep-/-) mouse. Am J Physiol Endocrinol Metab 298(6):E1236–E1243. https://doi.org/10.1152/ajpendo.00022.2010
Crielaard BJ, Lammers T, Rivella S (2017) Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov. https://doi.org/10.1038/nrd.2016.248
de Courten B, Jakubova M, de Courten MP, Kukurova IJ, Vallova S, Krumpolec P, Valkovic L, Kurdiova T, Garzon D, Barbaresi S, Teede HJ, Derave W, Krssak M, Aldini G, Ukropec J, Ukropcova B (2016) Effects of carnosine supplementation on glucose metabolism: pilot clinical trial. Obesity (Silver Spring) 24(5):1027–1034. https://doi.org/10.1002/oby.21434
Elbarbary NS, Ismail EAR, El-Naggar AR, Hamouda MH, El-Hamamsy M (2017) The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: a randomized placebo-controlled trial. Pediatr Diabetes. https://doi.org/10.1111/pedi.12564
Everaert I, Taes Y, De Heer E, Baelde H, Zutinic A, Yard B, Sauerhofer S, Vanhee L, Delanghe J, Aldini G, Derave W (2012) Low plasma carnosinase activity promotes carnosinemia after carnosine ingestion in humans. Am J Physiol Renal Physiol 302(12):F1537–F1544. https://doi.org/10.1152/ajprenal.00084.2012
Fernandez-Cao JC, Arija V, Aranda N, Basora J, Diez-Espino J, Estruch R, Fito M, Corella D, Salas-Salvado J (2017) Soluble transferrin receptor and risk of type 2 diabetes in the obese and nonobese. Eur J Clin Invest 47(3):221–230. https://doi.org/10.1111/eci.12725
Fernandez-Real JM, Ricart-Engel W, Arroyo E, Balanca R, Casamitjana-Abella R, Cabrero D, Fernandez-Castaner M, Soler J (1998) Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 21(1):62–68
Fernandez-Real JM, Lopez-Bermejo A, Ricart W (2002) Cross-talk between iron metabolism and diabetes. Diabetes 51(8):2348–2354
Fernandez-Real JM, Moreno JM, Lopez-Bermejo A, Chico B, Vendrell J, Ricart W (2007) Circulating soluble transferrin receptor according to glucose tolerance status and insulin sensitivity. Diabetes Care 30(3):604–608. https://doi.org/10.2337/dc06-1138
Fernandez-Real JM, Izquierdo M, Moreno-Navarrete JM, Gorostiaga E, Ortega F, Martinez C, Idoate F, Ricart W, Ibanez J (2009) Circulating soluble transferrin receptor concentration decreases after exercise-induced improvement of insulin sensitivity in obese individuals. Int J Obes (Lond) 33(7):768–774. https://doi.org/10.1038/ijo.2009.99
Freixenet N, Remacha A, Berlanga E, Caixas A, Gimenez-Palop O, Blanco-Vaca F, Bach V, Baiget M, Sanchez Y, Felez J, Gonzalez-Clemente JM (2009) Serum soluble transferrin receptor concentrations are increased in central obesity. Results from a screening programme for hereditary hemochromatosis in men with hyperferritinemia. Clin Chim Acta 400(1–2):111–116. https://doi.org/10.1016/j.cca.2008.10.019
Gillum RF (2001) Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 25(5):639–645. https://doi.org/10.1038/sj.ijo.0801561
Gong L, Yuan F, Teng J, Li X, Zheng S, Lin L, Deng H, Ma G, Sun C, Li Y (2014) Weight loss, inflammatory markers, and improvements of iron status in overweight and obese children. J Pediatr 164(4):795–800.e792. https://doi.org/10.1016/j.jpeds.2013.12.004
Heinegard D, Tiderstrom G (1973) Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta 43(3):305–310
Houjeghani S, Kheirouri S, Faraji E, Jafarabadi MA (2017) l-carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products and tumor necrosis factor alpha levels in patients with type 2 diabetes: a double-blind placebo-controlled randomized clinical trial. Nutr Res. https://doi.org/10.1016/j.nutres.2017.11.003
Johnson D, Bayele H, Johnston K, Tennant J, Srai SK, Sharp P (2004) Tumour necrosis factor alpha regulates iron transport and transporter expression in human intestinal epithelial cells. FEBS Lett 573(1–3):195–201. https://doi.org/10.1016/j.febslet.2004.07.081
Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J (2006) Iron deficiency in obese postmenopausal women. Obesity (Silver Spring) 14(10):1724–1730. https://doi.org/10.1038/oby.2006.198
Lecube A, Hernandez C, Pelegri D, Simo R (2008) Factors accounting for high ferritin levels in obesity. Int J Obes (Lond) 32(11):1665–1669. https://doi.org/10.1038/ijo.2008.154
Liu WH, Liu TC, Yin MC (2008) Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem Toxicol 46(5):1503–1509. https://doi.org/10.1016/j.fct.2007.12.013
Liu Q, Sun L, Tan Y, Wang G, Lin X, Cai L (2009) Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr Med Chem 16(1):113–129
Macdougall IC, Hutton RD, Cavill I, Coles GA, Williams JD (1989) Poor response to treatment of renal anaemia with erythropoietin corrected by iron given intravenously. BMJ 299(6692):157–158
Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG (1998) Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 44(1):45–51
Meng G, Yang H, Bao X, Zhang Q, Liu L, Wu H, Du H, Xia Y, Shi H, Guo X, Liu X, Li C, Su Q, Gu Y, Fang L, Yu F, Sun S, Wang X, Zhou M, Jia Q, Guo Q, Song K, Huang G, Wang G, Wu Y, Niu K (2017) Increased serum ferritin levels are independently related to incidence of prediabetes in adult populations. Diabetes Metab 43(2):146–153. https://doi.org/10.1016/j.diabet.2016.07.028
Montonen J, Boeing H, Steffen A, Lehmann R, Fritsche A, Joost HG, Schulze MB, Pischon T (2012) Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia 55(10):2613–2621. https://doi.org/10.1007/s00125-012-2633-y
Mozdzan M, Szemraj J, Rysz J, Nowak D (2005) Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin Pharmacol Toxicol 96(5):352–360. https://doi.org/10.1111/j.1742-7843.2005.pto_03.x
Nagai R, Murray DB, Metz TO, Baynes JW (2012) Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 61(3):549–559. https://doi.org/10.2337/db11-1120
Orban E, Schwab S, Thorand B, Huth C (2014) Association of iron indices and type 2 diabetes: a meta-analysis of observational studies. Diabetes Metab Res Rev 30(5):372–394. https://doi.org/10.1002/dmrr.2506
Oshiro S, Morioka MS, Kikuchi M (2011) Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv Pharmacol Sci 2011:378278. https://doi.org/10.1155/2011/378278
Peters V, Schmitt CP, Weigand T, Klingbeil K, Thiel C, van den Berg A, Calabrese V, Nawroth P, Fleming T, Forsberg E, Wagner AH, Hecker M, Vistoli G (2017) Allosteric inhibition of carnosinase (CN1) by inducing a conformational shift. J Enzyme Inhib Med Chem 32(1):1102–1110. https://doi.org/10.1080/14756366.2017.1355793
Podmore C, Meidtner K, Schulze MB, Scott RA, Ramond A, Butterworth AS, Di Angelantonio E, Danesh J, Arriola L, Barricarte A, Boeing H, Clavel-Chapelon F, Cross AJ, Dahm CC, Fagherazzi G, Franks PW, Gavrila D, Grioni S, Gunter MJ, Gusto G, Jakszyn P, Katzke V, Key TJ, Kuhn T, Mattiello A, Nilsson PM, Olsen A, Overvad K, Palli D, Quiros JR, Rolandsson O, Sacerdote C, Sanchez-Cantalejo E, Slimani N, Sluijs I, Spijkerman AM, Tjonneland A, Tumino R, van der DL A, van der Schouw YT, Feskens EJ, Forouhi NG, Sharp SJ, Riboli E, Langenberg C, Wareham NJ (2016) Association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC-interact study. Diabetes Care 39(4):572–581. https://doi.org/10.2337/dc15-0257
Price DL, Rhett PM, Thorpe SR, Baynes JW (2001) Chelating activity of advanced glycation end-product inhibitors. J Biol Chem 276(52):48967–48972. https://doi.org/10.1074/jbc.M108196200
Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB (2009a) The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 1790(7):671–681. https://doi.org/10.1016/j.bbagen.2008.04.005
Rajpathak SN, Negassa A, Kabat GC, Wylie-Rosett J, Rohan TE, Crandall J, Diabetes Prevention Program Research G (2009b) Elevated body iron stores predict the conversion from impaired glucose tolerance to type 2 diabetes. Diabetes Obes Metab 11(5):472–479. https://doi.org/10.1111/j.1463-1326.2008.00985.x
Regazzoni L, de Courten B, Garzon D, Altomare A, Marinello C, Jakubova M, Vallova S, Krumpolec P, Carini M, Ukropec J, Ukropcova B, Aldini G (2016) A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci Rep 6:27224. https://doi.org/10.1038/srep27224
Sharma AP, McKenna AM, Lepage N, Nieuwenhuys E, Filler G (2009) Relationships among serum iron, inflammation, and body mass index in children. Adv Pediatr 56:135–144. https://doi.org/10.1016/j.yapd.2009.08.014
Simcox JA, McClain DA (2013) Iron and diabetes risk. Cell Metab 17(3):329–341. https://doi.org/10.1016/j.cmet.2013.02.007
Skikne BS (2008) Serum transferrin receptor. Am J Hematol 83(11):872–875. https://doi.org/10.1002/ajh.21279
Soliman KM, Mohamed AM, Metwally NS (2007) Attenuation of some metabolic deteriorations induced by diabetes mellitus using carnosine. J Appl Sci 7:2252–2260
Souto JC, Remacha A, Buil A, Almasy L, Blangero J, Fontcuberta J (2003) Genetic determinants of iron metabolism plasma phenotypes and their relationship with risk of thrombosis. Haematologica 88(12):1436–1438
Speeckaert MM, Speeckaert R, Delanghe JR (2010) Biological and clinical aspects of soluble transferrin receptor. Crit Rev Clin Lab Sci 47(5–6):213–228. https://doi.org/10.3109/10408363.2010.550461
Tsai SJ, Kuo WW, Liu WH, Yin MC (2010) Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J Agric Food Chem 58(21):11510–11516. https://doi.org/10.1021/jf103258p
Tsuji Y, Miller LL, Miller SC, Torti SV, Torti FM (1991) Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts. Relationship to the induction of ferritin heavy chain. J Biol Chem 266(11):7257–7261
Tuomainen TP, Loft S, Nyyssonen K, Punnonen K, Salonen JT, Poulsen HE (2007) Body iron is a contributor to oxidative damage of DNA. Free Radic Res 41(3):324–328. https://doi.org/10.1080/10715760601091642
Velez S, Nair NG, Reddy VP (2008) Transition metal ion binding studies of carnosine and histidine: biologically relevant antioxidants. Colloids Surf B Biointerfaces 66(2):291–294. https://doi.org/10.1016/j.colsurfb.2008.06.012
Vlassara H (2001) The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 17(6):436–443
Yan SL, Wu ST, Yin MC, Chen HT, Chen HC (2009) Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci 74(8):H259–H265. https://doi.org/10.1111/j.1750-3841.2009.01330.x
Zhang S, Ntasis E, Kabtni S, van den Born J, Navis G, Bakker SJ, Kramer BK, Yard BA, Hauske SJ (2016) Hyperglycemia does not affect iron mediated toxicity of cultured endothelial and renal tubular epithelial cells: influence of l-carnosine. J Diabetes Res 2016:8710432. https://doi.org/10.1155/2016/8710432
Zheng Y, Li XK, Wang Y, Cai L (2008) The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin 32(1–2):135–145. https://doi.org/10.1080/03630260701727077
Acknowledgements
We thank the volunteers for their participation in the trial. We also thank Professor Wim Derave for performing the carnosinase measurements. This study was supported by the Grant Agency of the Slovak Academy of Sciences VEGA 2/0107/18, Slovak Research and Development Agency SRDA (APVV) 15/0253, Royal Australasian College of Physicians, Diabetes Australia Research Trust and Foundation for High Blood Pressure Research. Carnosine supplement was received from Flamma SPa, Italy. EB is a recipient of the Monash Graduate and Monash International Postgraduate Scholarships. BdC is supported by a National Heart Foundation Future Leader Fellowship (100864).
Author information
Authors and Affiliations
Contributions
EB conceived the study idea, performed data analysis and interpretation, wrote first draft of the manuscript and revised subsequent drafts until publication. BdC designed the study, contributed to the study idea and data analysis, and critically reviewed the manuscript. MPJdC co-designed the study with BdC and contributed to the review of the manuscript. JU & BU assisted with design of the study, performed all clinical measurement and contributed to review of the manuscript. TK & PK contributed to the clinical study performance and data collection. JMFR contributed to review of the manuscript. GA performed to laboratory measurement and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: S. P. Baba.
Rights and permissions
About this article
Cite this article
Baye, E., Ukropec, J., de Courten, M.P.J. et al. Carnosine supplementation reduces plasma soluble transferrin receptor in healthy overweight or obese individuals: a pilot randomised trial. Amino Acids 51, 73–81 (2019). https://doi.org/10.1007/s00726-018-2623-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2623-6