Abstract
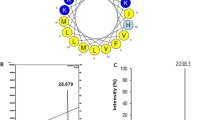
Melittin is the peptide toxin found in bee venom and is effective against cancer cells. To enhance its activity, a branched dimeric form of melittin was designed. The monomeric form of the peptide was more cytotoxic against gastric cancer cells at low concentrations (1–5 μM) than the dimer form, while the cytotoxic effect was comparable at higher concentrations (10 μM). Confocal microscopy showed that both the monomer and dimer forms of melittin with fluorescent label at the C terminus penetrated the cytoplasm and localized at the cell nucleus and disrupted the cell membrane. The results indicated that both peptides localized in the nucleus and no significant difference in penetration was observed between monomer and dimer of melittin. Although the C and N termini are important for melittin activity, using C terminus for dimerization of the peptide resulted in similar activity for the monomer and dimer against bacteria and gastric cancer cells.









Similar content being viewed by others
References
Barrera G (2012) Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012:21
Fields GB, Noble RL (1990) Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res 35(3):161–214
Giordano A, Cito L (2012) Advances in gastric cancer prevention. World J Clin Oncol 3(9):128–136
Gribenko AV, Guzman-Casado M, Lopez MM, Makhatadze GI (2002) Conformational and thermodynamic properties of peptide binding to the human S100P protein. Protein Sci 11(6):1367–1375
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8(21):2003–2014
Huang Y, Feng Q, Yan Q, Hao X, Chen Y (2015) Alpha-helical cationic anticancer peptides: a promising candidate for novel anticancer drugs. Mini Rev Med Chem 15(1):73–81
Iwadate M, Asakura T, Williamson MP (1998) The structure of the melittin tetramer at different temperatures—an NOE-based calculation with chemical shift refinement. Eur J Biochem 257(2):479–487
Jamasbi E, Batinovic S, Sharples RA, Sani MA, Robins-Browne RM, Wade JD, Separovic F, Hossain MA (2014) Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids 46(12):2759–2766
Jamasbi E, Ciccotosto GD, Tailhades J, Robins-Browne RM, Ugalde CL, Sharples RA, Patil N, Wade JD, Hossain MA, Separovic F (2015) Site of fluorescent label modifies interaction of melittin with live cells and model membranes. Biochimica et Biophysica Acta 1848(10 Pt A):2031–2039
Jamasbi E, Mularski A, Separovic F (2016) Model membrane and cell studies of antimicrobial activity of melittin analogues. Curr Top Med Chem 16(1):40–45
Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT (2012) Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol 258(1):72–81
Kohno M, Horibe T, Ohara K, Ito S, Kawakami K (2014) The membrane-lytic peptides K8L9 and melittin enter cancer cells via receptor endocytosis following subcytotoxic exposure. Chem Biol 21(11):1522–1532
Kong G-M, Tao W-H, Diao Y-L, Fang P-H, Wang J-J, Bo P, Qian F (2016) Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J Gastroenterol 22(11):3186–3195
Li W, O’Brien-Simpson NM, Tailhades J, Pantarat N, Dawson RM, Otvos L Jr, Reynolds EC, Separovic F, Hossain MA, Wade JD (2015) Multimerization of a proline-rich antimicrobial peptide, CHEX-Arg20, alters its mechanism of interaction with the Escherichia coli membrane. Chem Biol 22(9):1250–1258
Li W, O’Brien-Simpson NM, Yao S, Tailhades J, Reynolds EC, Dawson RM, Otvos L Jr, Hossain MA, Separovic F, Wade JD (2017) C-Terminal modification and multimerization increase the efficacy of a proline-rich antimicrobial peptide. Chemistry (Weinheim an der Bergstrasse, Germany) 23(2):390–396
Liu S, Yu M, He Y, Xiao L, Wang F, Song C, Sun S, Ling C, Xu Z (2008) Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology (Baltimore, Md) 47(6):1964–1973
Liu C-C, Hao D-J, Zhang Q, An J, Zhao J-J, Chen B, Zhang L-L, Yang H (2016) Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother Pharmacol 78(6):1113–1130
Miura Y (2012) NMR studies on the monomer-tetramer transition of melittin in an aqueous solution at high and low temperatures. Eur Biophys J 41(7):629–636
Orsolic N (2012) Bee venom in cancer therapy. Cancer Metastasis Rev 31(1–2):173–194
Othon CM, Kwon O-H, Lin MM, Zewail AH (2009) Solvation in protein (un)folding of melittin tetramer–monomer transition. Proc Natl Acad Sci 106(31):12593–12598
Qiu W, Zhang L, Kao YT, Lu W, Li T, Kim J, Sollenberger GM, Wang L, Zhong D (2005) Ultrafast hydration dynamics in melittin folding and aggregation: helix formation and tetramer self-assembly. J Phys Chem B 109(35):16901–16910
Rady I, Siddiqui IA, Rady M, Mukhtar H (2017) Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett 402:16–31
Raghuraman H, Chattopadhyay A (2007) Melittin: a membrane-active peptide with diverse functions. Biosci Rep 27(4–5):189–223
Ragin AD, Morgan RA, Chmielewski J (2002) Cellular Import mediated by nuclear localization signal peptide sequences. Chem Biol 9(8):943–948
Riss TL, Moravec RL, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L (2013) Cell viability assays. In: Sittampalam GS, Coussens NP, Brimacombe K, et al (eds) Assay guidance manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004. http://europepmc.org/books/NBK144065
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30
Sun D, Sun M, Zhu W, Wang Z, Li Y, Ma J (2015) The anti-cancer potency and mechanism of a novel tumor-activated fused toxin, DLM. Toxins 7(2):423–438
Terra RM, Guimaraes JA, Verli H (2007) Structural and functional behavior of biologically active monomeric melittin. J Mol Graph Model 25(6):767–772
Tosteson MT, Holmes SJ, Razin M, Tosteson DC (1985) Melittin lysis of red cells. J Membr Biol 87(1):35–44
van den Bogaart G, Guzmán JV, Mika JT, Poolman B (2008) On the mechanism of pore formation by melittin. J Biol Chem 283(49):33854–33857
Wu D, Yotnda P (2011) Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp JoVE 57:3357
Zhang D, Wang J, Xu D (2016) Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J Control Release 229((Supplement C)):130–139
Acknowledgements
FS acknowledges the Australian Research Council for award of grant DP140102127.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have potential conflicts of interest.
Research involving Human Participants and/or Animals
This research did not involve Human Participants and/or Animals and does not require Informed Consent.
Additional information
Handling Editor: F. Albericio.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 17685 kb)
Rights and permissions
About this article
Cite this article
Jamasbi, E., Lucky, S.S., Li, W. et al. Effect of dimerized melittin on gastric cancer cells and antibacterial activity. Amino Acids 50, 1101–1110 (2018). https://doi.org/10.1007/s00726-018-2587-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2587-6