Abstract
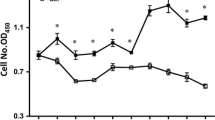
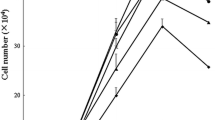
The synthetic dipeptides alanyl-glutamine (Ala-Gln) and glycyl-glutamine (Gly-Gln) are used as Gln substitution to provide energy source in the gastrointestinal tract due to their high solubility and stability. This study aimed to investigate the effects of Gln, Ala-Gln and Gly-Gln on mitochondrial respiration and protein turnover of enterocytes. Intestinal porcine epithelial cells (IPEC-J2) were cultured for 2 days in Dulbecco’s modified Eagle’s-F12 Ham medium (DMEM-F12) containing 2.5 mM Gln, Ala-Gln or Gly-Gln. Results from 5-ethynyl-2′-deoxyuridine incorporation and flow cytometry analysis indicated that there were no differences in proliferation between free Gln and Ala-Gln-treated cells, whereas Gly-Gln treatment inhibited the cell growth compared with Gln treatment. Significantly lower mRNA expressions of Sp1 and PepT1 were also observed in Gly-Gln-treated cells than that of Ala-Gln treatment. Ala-Gln treatment increased the basal respiration and ATP production, compared with free Gln and Gly-Gln treatments. There were no differences in protein turnover between free Gln and Ala-Gln-treated cells, but Gly-Gln treatment reduced protein synthesis and increased protein degradation. Ala-Gln treatment stimulated mTOR activation whereas Gly-Gln decreased mTOR phosphorylation and increased the UB protein expression compared with free Gln treatment. These results indicate that Ala-Gln has the very similar functional profile to free Gln in porcine enterocytes in vitro and can be substituted Gln as energy and protein sources in the gastrointestinal tract.






Similar content being viewed by others
Abbreviations
- 4EBP1:
-
4E binding protein-1
- AA:
-
Amino acids
- Ala-Gln:
-
Alanyl-glutamine
- ATP:
-
Adenosine triphosphate
- DMEM-F12:
-
Dulbecco’s modified Eagle’s-F12 Ham medium
- ECAR:
-
Extracellular acidification rate
- EdU:
-
5′-Ethynyl-2′-deoxyuridine
- FCCP:
-
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- Gln:
-
Glutamine
- Gly-Gln:
-
Glycyl-glutamine
- IPEC:
-
Intestinal porcine epithelial cell
- mTOR:
-
Mammalian target of rapamycin
- OCR:
-
Oxygen consumption rate
- PepT1:
-
Peptide transporter 1
- S6K1:
-
Ribosomal protein S6 kinase-1
- Sp1:
-
Specificity protein 1
- UB:
-
Ubiquitin
References
Ahmad S, White CW, Chang LY et al (2001) Glutamine protects mitochondrial structure and function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol 280:L779–L791
Aledo JC (2004) Glutamine breakdown in rapidly dividing cells: waste or investment? BioEssays 26:778–785
Braga-Neto MB, Oliveria BM, Rodrigues RS et al (2012) Protective effects of alanyl-glutamine supplementation against nelfinavir-induced epithelial impairment in IEC-6 cells and in mouse intestinal mucosa. Cancer Biol Ther 13:1482–1490
Brosnan JT (2003) Interorgan amino acid transport and its regulation. J Nutr 133:2068S–2072S
Cruzat VF, Tirapegui J (2009) Effects of oral supplementation with glutamine and alanyl-glutamine on glutamine, glutamate, and glutathione status in trained rats and subjected to long-duration exercise. Nutrition 25:428–435
Erickson RH, Kim YS (1990) Digestion and absorption of dietary protein. Annu Rev Med 41:133–139
Estivariz CF, Griffith DP, Luo M et al (2008) Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. J Parenter Enteral Nutr 32:389–402
Gilbert ER, Wong EA, Webb KE KE Jr. (2008) Board-invited review: peptide absorption and utilization: implications for animal nutrition and health. J Anim Sci 86:2135–2155
Haynes TE, Li P, Li X et al (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hu YJ, Smith DE, Ma K (2008) Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. J Mol Pharm 5:1122–1130
Jiang ZY, Sun LH, Lin YC et al (2009) Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J Anim Sci 87:4050–4056
Labow BI, Souba WW (2000) Glutamine. World J Surg 24:1503–1513
Lallès JP, Boudrya G, Faviera C et al (2004) Gut function and dysfunction in young pigs: physiology. Anim Res 53:301–316
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J Am Soc Nephrol 17:1807–1819
Li Y, Li J, Jiang J et al (2003) Glycyl-glutamine-supplemented long-term total parenteral nutrition selectively improves structure and function in heterotopic small-bowel autotransplantation in the pig. Transpl Int 16:866–871
Li GR, Li JJ, Tan BE et al (2015) Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets. PLoS One 10:e0128207
Matés JM, Segura JA, Campos-Sandoval JA et al (2009) Glutamine homeostasis and mitochondrial dynamics. Int J Biochem Cell Biol 41:2051–2061
Meissner B, Boll M, Daniel H et al (2004) Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem 279:36739–36745
Naka S, Saito H, Hashiguchi Y et al (1996) Alanyl-glutamine-supplemented total parenteral nutrition improves survival and protein metabolism in rat protracted bacterial peritonitis model. J Parenter Enteral Nutr 20:417–423
Newsholme P (2001) Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 131:2515S–2522S
Santos AA, Braga-Neto MB, Oliverira MR et al (2013) Glutamine and alanyl-glutamine increase RhoA expression and reduce Clostridium difficile toxin-a-induced intestinal epithelial cell damage. Biomed Res Int 2013:152052
Seglen PO, Gordon PB, Poli A (1980) Amino acid inhibition of the autophagic/lysosomal pathway of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta 630:103–118
Shimakura J, Terada T, Katsura T et al (2005) Characterization of the human peptide transporter PEPT1 promoter: Sp1 functions as a basal transcriptional regulator of human PEPT1. Am J Physiol Gastrointest Liver Physiol 289:G471–G477
Steinhardt HJ, Adibi SA (1986) Kinetics and characteristics of absorption from an equimolar mixture of 12 glycyl-dipeptides in human jejunum. Gastroenterology 90:577–582
Tan BE, Yin YL, Kong XF et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tan BE, Xiao H, Li FN et al (2015a) The profiles of mitochondrial respiration and glycolysis using extracellularflux analysis in porcine enterocyte IPEC-J2. Anim Nutr 1:239–243
Tan BE, Xiao H, Xiong X et al (2015b) l-Arginine improves DNA synthesis in LPS-challenged enterocytes. Front Biosci 20:989–1003
Wang H, Jia G, Huang L et al (2010) Study on the absorption and transport of different glutamine dipeptides in small intestine of weaned piglets. J Anim Plant Sci 7:751–759
Wang H, Jia G, Chen ZL et al (2011) The effect of glycyl-glutamine dipeptide concentration on enzyme activity, cell proliferation and apoptosis of jejunal tissues from weaned piglets. Agr Sci China 10:1088–1095
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433
Wu G, Bazer FW, Johnson GA et al (2011) TRIENNIAL GROWTH SYMPOSIUM: important roles for-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Yin YL, Yao K, Liu ZQ et al (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Yuneva M, Zamboni N, Oefner P et al (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178:93–105
Zhou YX, Zhang PS, Deng GG (2012) Improvements of immune status, intestinal integrity and gain performance in the early-weaned calves parenterally supplemented with l-alanyl-l-glutamine dipeptide. Vet Immunol Immunopathol 145:134–142
Acknowledgements
This study was in part supported by the National Natural Science Foundation of China (Nos. 31330075, 31372326, 31672433, 31301989 and 31560640), Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008) and National Basic Research Program of China (2013CB127302). We thank Changsha Lvye Biotechnology Limited Company Academician Expert Workstation, Guangdong Wangda Group Academician Workstation for Clean Feed Technology Research and Development in Swine, Guangdong Hinapharm Group Academician Workstation for Biological Feed and Feed Additives and Animal Intestinal Health for providing technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All authors listed have contributed to conception, design, gathering, analysis or interpretation of data and have contributed to the writing and intellectual content of the article. All authors gave informed consent to the submission of this manuscript.
Additional information
Handling Editors: C.-A.A. Hu, Y. Yin, Y. Hou, G. Wu, Y. Teng.
Rights and permissions
About this article
Cite this article
Tan, B., Liu, H., He, G. et al. Alanyl-glutamine but not glycyl-glutamine improved the proliferation of enterocytes as glutamine substitution in vitro. Amino Acids 49, 2023–2031 (2017). https://doi.org/10.1007/s00726-017-2460-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2460-z