Abstract
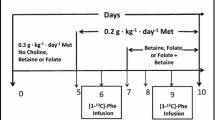
Methionine metabolism is critical during development with significant requirements for protein synthesis and transmethylation reactions. However, separate requirements of methionine for protein synthesis and transmethylation are difficult to define because after transmethylation, demethylated methionine is either irreversibly oxidized to cysteine during transsulfuration, or methionine is regenerated by the dietary methyl donors, choline (via betaine) or folate during remethylation. We hypothesized that remethylation contributes significantly to methionine availability and affects partitioning between protein and transmethylation. 4–8-day-old neonatal piglets were fed a diet devoid (MD−) (n = 8) or replete (MS+) (n = 8) of folate, choline and betaine to limit remethylation. After 5 days, dietary methionine was reduced to 80 % of requirement in both groups of piglets to ensure methionine availability was limited. On day 7, an intragastric infusion of [13C1]methionine and [2H3-methyl]methionine was administered to measure methionine cycle flux. In MD− piglets, in vivo remethylation was 60 % lower despite 23-fold greater conversion of choline to betaine (P < 0.05) and transmethylation was 56 % lower (P < 0.05), suggesting dietary methyl donors spared 425 µmol methyl/day for transmethylation. The priority of protein synthesis versus transmethylation was clear during MD− feeding (P < 0.05), as an additional 6 % of methionine flux was for protein synthesis in those piglets (P < 0.05). However, whole body transsulfuration was unaffected in vivo despite reduced in vitro cystathionine-β-synthase capacity in MD− piglets (P < 0.05). Our data show that remethylation contributes significantly to methionine availability and that transmethylation is sacrificed to maintain protein synthesis when methionine is limiting in neonates, which should be considered when determining the methionine requirement.

Similar content being viewed by others
Abbreviations
- APE:
-
Atom percent excess
- BHMT:
-
Betaine:homocysteine methyltransferase
- CBS:
-
Cystathionine-β-synthase
- DMG:
-
Dimethylglycine
- FNC:
-
Fractional net conversion
- FPF:
-
Fraction of product flux
- MSyn:
-
Methionine synthase
- Q :
-
Flux
References
Albanese AA, Holt LE Jr, Davis VI, Snyderman SE, Lein M, Smetak EM (1949) The sulfur amino acid requirement of the infant. J Nutr 37:511–520
Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG (2009) Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296:E1239–E1250. doi:10.1152/ajpendo.91021.2008
Bertolo RF, McBreairty LE (2013) The nutritional burden of methylation reactions. Curr Opin Clin Nutr Metab Care 16:102–108. doi:10.1097/MCO.0b013e32835ad2ee
Bertolo RFP, Brunton JA, Pencharz PB, Ball RO (2003) Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284:E915–E922. doi:10.1152/ajpendo.00269.2002
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using pre-column derivatization. J Chromatogr 336:93–104
Brosnan JT, Brosnan ME, Bertolo RF, Brunton JA (2007) Methionine: a metabolically unique amino acid. Livestock Sci 112:2–7. doi:10.1016/j.livsci.2007.07.005
Brunton JA, Baldwin MP, Hanna RA, Bertolo RF (2012) Proline supplementation to parenteral nutrition results in greater rates of protein synthesis in the muscle, skin, and small intestine in neonatal Yucatan miniature piglets. J Nutr 142:1004–1008. doi:10.3945/jn.111.154534
Clarke JT, Bier DM (1982) The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metab Clin Exp 31:999–1005
Clow KA, Treberg JR, Brosnan ME, Brosnan JT (2008) Elevated tissue betaine contents in developing rats are due to dietary betaine, not to synthesis. J Nutr 138:1641–1646
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ (1994) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132
Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF 3rd (2005) Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr 81:648–655
Engel RW (1943) The choline content of animal and plant products. J Nutr 25:441–446
Finkelstein JD, Mudd SH (1967) Trans-sulfuration in mammals. The methionine-sparing effect of cystine. J Biol Chem 242:873–880
Garrow TA (1996) Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem 271:22831–22838. doi:10.1074/jbc.271.37.22831
Gornall GA, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Holm PI, Ueland PM, Kvalheim G, Lien EA (2003) Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49:286–294
House JD, Pencharz PB, Ball RO (1997) Phenylalanine requirements determined by using L-[1-14C]phenylalanine in neonatal piglets receiving total parenteral nutrition supplemented with tyrosine. Am J Clin Nutr 65:984–993
Huang L, Hogewind-Schoonenboom JE, van Dongen MJ, de Groof F, Voortman GJ, Schierbeek H, Twisk JW, Vermes A, Chen C, Huang Y, van Goudoever JB (2012) Methionine requirement of the enterally fed term infant in the first month of life in the presence of cysteine. Am J Clin Nutr 95:1048–1054. doi:10.3945/ajcn.111.028779
Kirsch SH, Herrmann W, Rabagny Y, Obeid R (2010) Quantification of acetylcholine, choline, betaine, and dimethylglycine in human plasma and urine using stable-isotope dilution ultra performance liquid chromatography–tandem mass spectrometry. J Chrom B 878:3338–3344. doi:10.1016/j.jchromb.2010.10.016
Koblin DD, Watson JE, Deady JE, Stokstad EL, Eger EI 2nd (1981) Inactivation of methionine synthetase by nitrous oxide in mice. Anesthesiology 54:318–324
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
MacCoss MJ, Fukagawa NK, Matthews DE (2001) Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab 280:E947–E955
MacKay DS, Brophy JD, McBreairty LE, McGowan RA, Bertolo RF (2012) Intrauterine growth restriction leads to changes in sulfur amino acid metabolism, but not global DNA methylation, in Yucatan miniature piglets. J Nutr Biochem 23:1121–1127. doi:10.1016/j.jnutbio.2011.06.005
Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S (2008) Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem 19:207–215
Mudd SH, Poole JR (1975) Labile methyl balances for normal humans on various dietary regimens. Metab Clin Exp 24:721–735
National Research Council (2012) Nutrient requirements of swine, 11th edn. National Academies Press, Washington. doi:10.17226/13298
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Riedijk MA, Stoll B, Chacko S, Schierbeek H, Sunehag AL, van Goudoever JB, Burrin DG (2007) Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci USA 104:3408–3413. doi:10.1073/pnas.0607965104
Robinson JL, McBreairty LE, Harding SV, Randell EW, Brunton JA, Bertolo RF (2014) The dietary methyl donors folate, betaine and choline have a significant impact on the partitioning of methionine in the neonatal piglet. Appl Physiol Nutr Metab 39:635. doi:10.1139/apnm-2014-0085
Schneider WJ, Vance DE (1978) Effect of choline deficiency on the enzymes that synthesize phosphatidylcholine and phosphatidylethanolamine in rat liver. Eur J Biochem 85:181–187
Schubert HL, Blumenthal RM, Cheng X (2003) Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci 28:329–335. doi:10.1016/S0968-0004(03)00090-2
Shoveller AK, Brunton JA, House JD, Pencharz PB, Ball RO (2003a) Dietary cysteine reduces the methionine requirement by an equal proportion in both parenterally and enterally fed piglets. J Nutr 133:4215–4224
Shoveller AK, Brunton JA, Pencharz PB, Ball RO (2003b) The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J Nutr 133:1390–1397
Shoveller AK, House JD, Brunton JA, Pencharz PB, Ball RO (2004) The balance of dietary sulfur amino acids and the route of feeding affect plasma homocysteine concentrations in neonatal piglets. J Nutr 134:609–612
Storch KJ, Wagner DA, Burke JF, Young VR (1988) Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol 255:E322–E331
Storch KJ, Wagner DA, Burke JF, Young VR (1990) [1-13C; methyl-2H3]methionine kinetics in humans: methionine conservation and cystine sparing. Am J Physiol 258:E790–E798
Storch KJ, Wagner DA, Young VR (1991) Methionine kinetics in adult men: effects of dietary betaine on L-[2H3-methyl-1-13C]methionine. Am J Clin Nutr 54:386–394
Tang Y, Tan B, Xiong X, Li F, Ren W, Kong X, Qiu W, Hardwidge PR, Yin Y (2015) Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids 47:2199–2204. doi:10.1007/s00726-014-1781-4
Taoka S, Ohja S, Shan X, Kruger WD, Banerjee R (1998) Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem 273:25179–25184. doi:10.1074/jbc.273.39.25179
Thompson GN, Pacy PJ, Merritt H, Ford GC, Read MA, Cheng KN, Halliday D (1989) Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 256:E631–E639
Urschel KL, Rafii M, Pencharz PB, Ball RO (2007) A multitracer stable isotope quantification of the effects of arginine intake on whole body arginine metabolism in neonatal piglets. Am J Physiol Endocrinol Metab 293:E811–E818. doi:10.1152/ajpendo.00290.2007
Vance DE, Li Z, Jacobs RL (2007) Hepatic phosphatidylethanolamine N-methyltransferase, unexpected roles in animal biochemistry and physiology. J Biol Chem 282:33237–33241. doi:10.1074/jbc.R700028200
Vester B, Rasmussen K (1991) High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem 29:549–554
Walkey CJ, Yu L, Agellon LB, Vance DE (1998) Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem 273:27043–27046. doi:10.1074/jbc.273.42.27043
Weinhold PA, Sanders R (1973) The oxidation of choline by liver slices and mitochondria during liver development in the rat. Life Sci 13:621–629. doi:10.1016/0024-3205(73)90055-6
Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A (1991) Choline, an essential nutrient for humans. FASEB J 5:2093–2098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding disclosure
This project was funded with support from the Canadian Institutes of Health Research (Grant number 201103RNL) and the Research & Development Corporation of Newfoundland and Labrador (Grant number 5404-1046-104) awarded to RFB. JLR was supported by a fellowship from the Canadian Institutes of Health Research and the Research & Development Corporation of Newfoundland and Labrador.
Conflict of interest
JLR, LEM, EWR, JAB, RFB declare no conflicts of interest.
Additional information
Handling Editor: C.-A. A. Hu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robinson, J.L., Bartlett, R.K., Harding, S.V. et al. Dietary methyl donors affect in vivo methionine partitioning between transmethylation and protein synthesis in the neonatal piglet. Amino Acids 48, 2821–2830 (2016). https://doi.org/10.1007/s00726-016-2317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2317-x