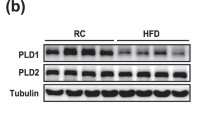
Abstract
Although it is known that a low-protein diet induces hepatic triglyceride (TG) accumulation in both rodents and humans, little is known about the underlying mechanism. In the present study, we modeled hepatic TG accumulation by inducing dietary protein deficiency in mice and aimed to determine whether certain amino acids could prevent low-protein diet-induced TG accumulation in the mouse liver. Mice fed a diet consisting of 3 % casein (3C diet) for 7 days showed hepatic TG accumulation with up-regulation of TG synthesis for the Acc gene and down-regulation of TG-rich lipoprotein secretion from hepatocytes for Mttp genes. Supplementing the 3 % casein diet with essential amino acids, branched-chain amino acids, or the single amino acid leucine rescued hepatic TG accumulation. In the livers of mice fed the 3 % casein diet, we observed a decrease in the levels of the autophagy substrate p62, an increase in the expression levels of the autophagy marker LC3-II, and an increase in the splicing of the endoplasmic reticulum (ER) stress-dependent Xbp1 gene. Leucine supplementation to the 3 % casein diet did not affect genes related to lipid metabolism, but inhibited the decrease in p62, the increase in LC3-II, and the increase in Xbp1 splicing levels in the liver. Our results suggest that ER stress responses and activated autophagy play critical roles in low-protein diet-induced hepatic TG accumulation in mice, and that leucine suppresses these two major protein degradation systems. This study contributes to understanding the mechanisms of hepatic disorders of lipid metabolism.






Similar content being viewed by others
References
Aoki N, Yoshida D, Ishikawa R, Ando M, Nakamura K, Tahara Y et al (2014) A single daily meal at the beginning of the active or inactive period inhibits food deprivation-induced fatty liver in mice. Nutr Res 34:613–622
Baena M, Sangüesa G, Hutter N, Sánchez RM, Roglans N, Laguna JC et al (2015) Fructose supplementation impairs rat liver autophagy through mTORC activation without inducing endoplasmic reticulum stress. Biochim Biophys Acta 1851:107–116
Blouet C, Jo YH, Li X, Schwartz GJ (2009) Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 29:8302–8311
Campos-Ferraz PL, Bozza T, Nicastro H, Lancha AH Jr (2013) Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 29:1388–1394
Carallo C, Mancuso G, Mauro G, Laghi F, Madafferi B, Irace C et al (2009) Hepatic steatosis, carotid atherosclerosis and metabolic syndrome: the STEATO Study. J Gastroenterol 44:1156–1161
Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S et al (2010) Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 59:17–25
Cunard R (2015) Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med 4:715–740
Deldicque L, Sanchez Canedo C, Horman S, De Potter I, Bertrand L, Hue L et al (2008) Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids 35:147–155
Dodd KM, Tee AR (2012) Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 302:E1329–E1342
Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A et al (2011) p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell 44:134–146
Flores H, Sierralta W, Monckeberg F (1970) Triglyceride transport in protein-depleted rats. J Nutr 100:375–379
Fung TS, Liao Y, Liu DX (2014) The endoplasmic reticulum stress sensor IRE1α protects cells from apoptosis induced by the coronavirus infectious bronchitis virus. J Virol 88:12752–12764
Fuse Y, Hirao A, Kuroda H, Otsuka M, Tahara Y, Shibata S (2012) Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J Circadian Rhythms 10:4
Garcia-Caraballo SC, Comhair TM, Verheyen F, Gaemers I, Schaap FG, Houten SM et al (2013) Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim Biophys Acta 1832:685–695
Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T et al (2011) Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem 112:30–38
Hinault C, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E (2004) Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J 18:1894–1896
Hooper AJ, Adams LA, Burnett JR (2011) Genetic determinants of hepatic steatosis in man. J Lipid Res 52:593–617
Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20:1981–1991
Hosomi S, Kaser A, Blumberg RS (2015) Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol 31:81–88
Inami Y, Yamashina S, Izumi K, Ueno T, Tanida I, Ikejima K et al (2011) Hepatic steatosis inhibits autophagic proteolysis via impairment of autophagosomal acidification and cathepsin expression. Biochem Biophys Res Commun 412:618–625
Karademir B, Corek C, Ozer NK (2015) Endoplasmic reticulum stress and proteasomal system in amyotrophic lateral sclerosis. Free Radic Biol Med S0891–5849(15):00261
Kawaguchi T, Izumi N, Charlton MR, Sata M (2011) Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 54:1063–1070
Komatsu H, Nishihira T, Chin M, Doi H, Shineha R, Mori S et al (1997) Effects of caloric intake on anticancer therapy in rats with valine-depleted amino acid imbalance. Nutr Cancer 28:107–112
Komatsu H, Nishihira T, Chin M, Doi H, Shineha R, Mori S et al (1998) Effect of valine-depleted total parenteral nutrition on fatty liver development in tumor-bearing rats. Nutrition 14:276–281
Latorraca MQ, Reis MA, Carneiro EM, Mello MA, Velloso LA, Saad MJ et al (1998) Protein deficiency and nutritional recovery modulate insulin secretion and the early steps of insulin action in rats. J Nutr 128:1643–1649
Liu Y, Song H, Wang L, Xu H, Shu X, Zhang L et al (2014) Hepatoprotective and antioxidant activities of extracts from Salvia-Nelumbinis naturalis against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet in mice. J Transl Med 12:315
Lívero FA, Stolf AM, Dreifuss AA, Bastos-Pereira AL, Chicorski R, de Oliveira LG et al (2014) The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact 217:19–27
Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H et al (2014) Role of hypoxia inducing factor-1β in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS ONE 9:e115849
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Ota T, Gayet C, Ginsberg HN (2008) Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118:316–332
Ozaki Y, Takeda T, Akanishi N, Hakuno F, Toyoshima Y, Takahashi S et al (2014) Insulin injection restored increased insulin receptor substrate (IRS)-2 protein during short-term protein restriction but did not affect reduced insulin-like growth factor (IGF)-I mRNA or increased triglyceride accumulation in the liver of rats. Biosci Biotechnol Biochem 78:130–138
Pösö AR, Wert JJ Jr, Mortimore GE (1982) Multifunctional control of amino acids of deprivation-induced proteolysis in liver. Role of leucine. J Biol Chem 257:12114–12120
Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R (2010) Autophagy in liver diseases. J Hepatol 53:1123–1134
Rennie MJ, Bohé J, Smith K, Wackerhage H, Greenhaff P (2006) Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr 136:264S–268S
Samali A, Fitzgerald U, Deegan S, Gupta S (2010) Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol 2010:830307
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M et al (2009) Autophagy regulates lipid metabolism. Nature 458:1131–1135
Toyoshima Y, Tokita R, Ohne Y, Hakuno F, Noguchi T, Minami S et al (2010) Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J Mol Endocrinol 45:329–340
van Schadewijk A, van’t Wout EF, Stolk J, Hiemstra PS (2012) A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones 17:275–279
Verfaillie T, Salazar M, Velasco G, Agostinis P et al (2010) Linking ER stress to autophagy: potential implications for cancer therapy. Int J Cell Biol 2010:930509
Wilgram GF, Lucas CC, Best CH (1958) Kwashiorkor type of fatty liver in primates. J Exp Med 108:361–370
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010) Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11:467–478
Yokota S, Nakamura K, Ando M, Kamei H, Hakuno F, Takahashi S et al (2014) Acetylcholinesterase (AChE) inhibition aggravates fasting-induced triglyceride accumulation in the mouse liver. FEBS Open Bio 4:905–914
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH (2007) Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 56:1647–1654
Acknowledgments
This work was partially supported by the Council for Science, Technology and Innovation, SIP, “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO) (S.S.); by a Grant-in-Aid for Scientific Research (S) (26220201) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (S.S.); and by a Grant-in-Aid for Scientific Research (C) (15K07740) from the Japan Society for the Promotion of Science (S.Y.). No additional external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest, financial or otherwise.
Additional information
Handling Editor: C.-A. A. Hu.
S.-I. Yokota and M. Ando contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yokota, SI., Ando, M., Aoyama, S. et al. Leucine restores murine hepatic triglyceride accumulation induced by a low-protein diet by suppressing autophagy and excessive endoplasmic reticulum stress. Amino Acids 48, 1013–1021 (2016). https://doi.org/10.1007/s00726-015-2149-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2149-0