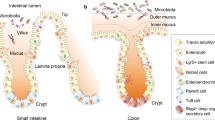
Abstract
The intestinal barrier integrity is essential for the absorption of nutrients and health in humans and animals. Dysfunction of the mucosal barrier is associated with increased gut permeability and development of multiple gastrointestinal diseases. Recent studies highlighted a critical role for glutamine, which had been traditionally considered as a nutritionally non-essential amino acid, in activating the mammalian target of rapamycin cell signaling in enterocytes. In addition, glutamine has been reported to enhance intestinal and whole-body growth, to promote enterocyte proliferation and survival, and to regulate intestinal barrier function in injury, infection, weaning stress, and other catabolic conditions. Mechanistically, these effects were mediated by maintaining the intracellular redox status and regulating expression of genes associated with various signaling pathways. Furthermore, glutamine stimulates growth of the small intestinal mucosa in young animals and also enhances ion transport by the gut in neonates and adults. Growing evidence supports the notion that glutamine is a nutritionally essential amino acid for neonates and a conditionally essential amino acid for adults. Thus, as a functional amino acid with multiple key physiological roles, glutamine holds great promise in protecting the gut from atrophy and injury under various stress conditions in mammals and other animals.



Similar content being viewed by others
Abbreviations
- ASCT2:
-
Neutral amino acid transporter type 2
- ATP:
-
Adenosine triphosphate
- BSO:
-
Buthionine sulfoximine
- DP:
-
Dipeptidase
- EAA:
-
Essential amino acids
- 4EBP1:
-
Eukaryotic translation initiation factor 4E-binding protein-1
- eIF4E:
-
Eukaryotic translation initiation factor 4E
- FOXO:
-
Forkhead box O transcription factor
- GA:
-
GlutaminaseGALT gut-associated lymphatic tissue
- GCL:
-
Glutamate cysteine ligase
- GS:
-
Glutamine synthetase
- GSH:
-
Glutathione
- GSS:
-
Glutathione synthetase
- GSSG:
-
Glutathione disulfide
- γ-GT:
-
γ-Glutamyl transpeptidase
- Ig:
-
Immunoglobulin
- IκB:
-
Inhibitor of NF-κB
- α-KG:
-
α-Ketoglutarate
- IUGR:
-
Intrauterine growth restriction
- MAPK:
-
Mitogen-activated protein kinases
- MS:
-
Methionine sulfoximine
- mTOR:
-
Mammalian target of rapamycin
- mTORC:
-
mTOR complex
- NADH:
-
Reduced form of nicotinamide adenine dinucleotide
- NADPH:
-
Reduced form of nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- p70S6k:
-
p70 Ribosomal protein S6 kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- PKB/AKT:
-
Protein kinase B
- PPP:
-
Pentose phosphate pathway
- Rheb:
-
Ras homologue enriched in brain
- ROS:
-
Reactive oxygen species
- RTK:
-
Receptor tyrosine kinase
- SNAT2:
-
Sodium coupled neutral amino acid transporter 2
- TCL:
-
Tricarboxylic acid cycle
- TNF-α:
-
Tumor necrosis factor-alpha
- TPN:
-
Total parenteral nutrition
- Trx:
-
Reduced thioredoxin
- TrxSS:
-
Oxidized thioredoxin
- TSC:
-
Tuberous sclerosis complex
- ZO-1:
-
Zonula occludens protein 1
References
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144
Alverdy JC (1990) Effects of glutamine-supplemented diets on immunology of the gut. JPEN J Parenter Enteral Nutr 14:109S–113S
Alverdy JC, Aoys E, Moss GS (1988) Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 104:185–190
Arrieta MC, Bistritz L, Meddings JB (2006) Alterations in intestinal permeability. Gut 55:1512–1520
Artis D (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8:411–420
Avissar NE, Sax HC, Toia L (2008) In human entrocytes, GLN transport and ASCT2 surface expression induced by short-term EGF are MAPK, PI3 K, and Rho-dependent. Dig Dis Sci 53:2113–2125
Bach SP, Renehan AG, Potten CS (2000) Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis 21:469–476
Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y et al (2007) NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol 41:447–460
Barker N, van de Wetering M, Clevers H (2008) The intestinal stem cell. Genes Dev 22:1856–1864
Basuroy S, Sheth P, Mansbach CM, Rao RK (2005) Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and l-glutamine. Am J Physiol Gastrointest Liver Physiol 289:G367–G375
Booth C, Evans GS, Potten CS (1995) Growth factor regulation of proliferation in primary cultures of small intestinal epithelium. In Vitro Cell Dev Biol Anim 31:234–243
Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD et al (1995) Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr 19:453–460
Burke DJ, Alverdy JC, Aoys E, Moss GS (1989) Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg 124:1396–1399
Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24:503–512
Campbell JM, Crenshaw JD, Polo J (2013) The biological stress of early weaned piglets. J Anim Sci Biotechnol 4:19
Carneiro BA, Fujii J, Brito GA, Alcantara C, Oria RB, Lima AA et al (2006) Caspase and bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect Immun 74:81–87
Chen K, Okuma T, Okamura K, Torigoe Y, Miyauchi Y (1994) Glutamine-supplemented parenteral nutrition improves gut mucosa integrity and function in endotoxemic rats. JPEN J Parenter Enteral Nutr 18:167–171
Chen Y, Li DF, Dai ZL et al (2014) L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning pigs. Amino Acids 46:1131–1142
Circu ML, Aw TY (2011) Redox biology of the intestine. Free Radical Res 45:1245–1266
Circu ML, Aw TY (2012) Glutathione and modulation of cell apoptosis. Biochim Biophys Acta 1823:1767–1777
Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL (2003) Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut 52:224–230
Coeffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotte P et al (2002) Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr 132:2570–2573
Crespo I, San-Miguel B, Prause C, Marroni N, Cuevas MJ, Gonzalez-Gallego J et al (2012) Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS One 7:e50407
Crowe SE, Perdue MH (1992) Functional abnormalities in the intestine associated with mucosal mast cell activation. Reg Immunol 4:113–117
Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC et al (2005) Molecular mechanisms of glutamine action. J Cell Physiol 204:392–401
Dai ZL, Zhang J, Wu G, Zhu WY (2010) Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 39:1201–1215
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2012) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY (2013) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
de Santa Barbara P, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60:1322–1332
DeMarco VG, Li N, Thomas J, West CM, Neu J (2003) Glutamine and barrier function in cultured Caco-2 epithelial cell monolayers. J Nutr 133:2176–2179
Deniel N, Marion-Letellier R, Charlionet R, Tron F, Leprince J, Vaudry H et al (2007) Glutamine regulates the human epithelial intestinal HCT-8 cell proteome under apoptotic conditions. Mol Cell Proteomics 6:1671–1679
D’Inca R, Kloareg M, Gras-Le Guen C, Le Huerou-Luron I (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140:925–931
D’Inca R, Gras-Le Guen C, Che L, Sangild PT, Le Huerou-Luron I (2011) Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 99:208–216
Evans ME, Jones DP, Ziegler TR (2003) Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr 133:3065–3071
Evans ME, Jones DP, Ziegler TR (2005) Glutamine inhibits cytokine-induced apoptosis in human colonic epithelial cells via the pyrimidine pathway. Am J Physiol Gastrointest Liver Physiol 289:G388–G396
Farhadi A, Banan A, Fields J, Keshavarzian A (2003) Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 18:479–497
Findley MK, Koval M (2009) Regulation and roles for claudin-family tight junction proteins. IUBMB Life 61:431–437
Franco R, Cidlowski JA (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16:1303–1314
Furuse M, Tsukita S (2006) Claudins in occluding junctions of humans and flies. Trends Cell Biol 16:181–188
Gareau MG, Jury J, Perdue MH (2007) Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 293:G198–G203
Grant JP, Snyder PJ (1988) Use of l-glutamine in total parenteral nutrition. J Surg Res 44:506–513
Groschwitz KR, Hogan SP (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124:3–20 (quiz 21-22)
Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M et al (2009) Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci 106:22381–22386
Han X, Ren X, Jurickova I, Groschwitz K, Pasternak BA, Xu H et al (2009) Regulation of intestinal barrier function by signal transducer and activator of transcription 5b. Gut 58:49–58
Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE et al (2009) l-Glutamine or L-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hou Y, Yao K, Wang L, Ding B, Fu D, Liu Y et al (2011) Effects of alpha-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br J Nutr 106:357–363
Jacobi SK, Odle J (2012) Nutritional factors influencing intestinal health of the neonate. Adv Nutr 3:687–696
Jiang ZY, Sun LH, Lin YC, Ma XY, Zheng CT, Zhou GL et al (2009) Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J Anim Sci 87:4050–4056
Kern JC, Kehrer JP (2005) Free radicals and apoptosis: relationships with glutathione, thioredoxin, and the BCL family of proteins. Front Biosci 10:1727–1738
Kourtis N, Tavernarakis N (2009) Autophagy and cell death in model organisms. Cell Death Differ 16:21–30
Larson SD, Li J, Chung DH, Evers BM (2007) Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol 293:G1262–G1271
Lesueur C, Bole-Feysot C, Bekri S, Husson A, Lavoinne A, Brasse-Lagnel C (2012) Glutamine induces nuclear degradation of the NF-kappaB p65 subunit in Caco-2/TC7 cells. Biochimie 94:806–815
Li N, Neu J (2009) Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 139:710–714
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–734
Li N, Lewis P, Samuelson D, Liboni K, Neu J (2004) Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol 287:G726–G733
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li P, Knabe DA, Kim SW et al (2009) Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Liboni KC, Li N, Scumpia PO, Neu J (2005) Glutamine modulates LPS-induced IL-8 production through IkappaB/NF-kappaB in human fetal and adult intestinal epithelium. J Nutr 135:245–251
Marc Rhoads J, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Mates JM, Perez-Gomez C, Nunez de Castro I, Asenjo M, Marquez J (2002) Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol 34:439–458
Mates JM, Segura JA, Alonso FJ, Marquez J (2006) Pathways from glutamine to apoptosis. Front Biosci 11:3164–3180
Matter K, Balda MS (2003) Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4:225–236
Meadows AL, Kong B, Berdichevsky M, Roy S, Rosiva R, Blanch HW et al (2008) Metabolic and morphological differences between rapidly proliferating cancerous and normal breast epithelial cells. Biotechnol Prog 24:334–341
Menard S, Cerf-Bensussan N, Heyman M (2010) Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 3:247–259
Mestecky J, Russell MW, Jackson S, Brown TA (1986) The human IgA system: a reassessment. Clin Immunol Immunopathol 40:105–114
Moeser AJ, Ryan KA, Nighot PK, Blikslager AT (2007) Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol 293:G413–G421
Mondello S, Galuppo M, Mazzon E, Domenico I, Mondello P, Carmela A et al (2010) Glutamine treatment attenuates the development of ischaemia/reperfusion injury of the gut. Eur J Pharmacol 643:304–315
Musch MW, Hayden D, Sugi K, Straus D, Chang EB (1998) Cell-specific induction of hsp72-mediated protection by glutamine against oxidant injury in IEC18 cells. Proc Assoc Am Physicians 110:136–139
Neu J, DeMarco V, Weiss M (1999) Glutamine supplementation in low-birth-weight infants: mechanisms of action. JPEN J Parenter Enteral Nutr 23:S49–S51
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B et al (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534
Noth R, Hasler R, Stuber E, Ellrichmann M, Schafer H, Geismann C et al (2013) Oral glutamine supplementation improves intestinal permeability dysfunction in a murine acute graft-vs.-host disease model. Am J Physiol Gastrointest Liver Physiol 304:G646–G654
Papaconstantinou HT, Hwang KO, Rajaraman S, Hellmich MR, Townsend CM Jr, Ko TC (1998) Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery 124:152–159 (discussion 159-160)
Pasparakis M (2009) Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol 9:778–788
Pasparakis M (2012) Role of NF-kappaB in epithelial biology. Immunol Rev 246:346–358
Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT et al (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85:1139–1162
Radtke F, Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307:1904–1909
Ramsay DB, Stephen S, Borum M, Voltaggio L, Doman DB (2010) Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y) 6:772–777
Reeds PJ, Burrin DG, Stoll B, Jahoor F, Wykes L, Henry J et al (1997) Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am J Physiol 273:E408–E415
Ren WK, Luo W, Wu MM et al (2013a) Dietary L-glutamine supplementation improves pregnancy outcome in mice infected with type 2 porcine circovirus. Amino Acids 45:479–488
Ren WK, Liu SP, Chen S et al (2013b) Dietary L-glutamine supplementation increases pasteurella multocida burden and expression of major virulence factors. Amino Acids 45:947–955
Rezaei R, Knabe DA, Li XL et al (2011) Enhanced efficiency of milk utilization for growth in surviving low-birth-weight piglets. J Anim Sci Biotechnol 2:73–83
Rezaei R, Wang WW, Wu ZL et al (2013a) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol 4:7
Rezaei R, Knabe DA, Tekwe CD et al (2013b) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rhoads M (1999) Glutamine signaling in intestinal cells. JPEN J Parenter Enteral Nutr 23:S38–S40
Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD et al (1997) l-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol 272:G943–G953
Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB (2005) Anti-apoptotic effects of l-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 129:170–184
Ruth MR, Field CJ (2013) the immune modifying effects of the gut-associated lymphoid tissue. J Anim Sci Biotechnol 4:27
Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB (2009) Glutamine increases autophagy under basal and stressed conditions in intestinal epithelial cells. Gastroenterology 136:924–932
Sandri M (2012) FOXOphagy path to inducing stress resistance and cell survival. Nat Cell Biol 14:786–788
Scheppach W, Dusel G, Kuhn T, Loges C, Karch H, Bartram HP et al (1996) Effect of l-glutamine and n-butyrate on the restitution of rat colonic mucosa after acid induced injury. Gut 38:878–885
Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286:C1213–C1228
Schroder J, Wardelmann E, Winkler W, Fandrich F, Schweizer E, Schroeder P (1995) Glutamine dipeptide-supplemented parenteral nutrition reverses gut atrophy, disaccharidase enzyme activity, and absorption in rats. JPEN J Parenter Enteral Nutr 19:502–506
Seth A, Basuroy S, Sheth P, Rao RK (2004) l-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287:G510–G517
Shaker A, Rubin DC (2010) Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res 156:180–187
Sheard NF, Walker WA (1988) The role of breast milk in the development of the gastrointestinal tract. Nutr Rev 46:1–8
Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE et al (2010) Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol 298:G352–G363
Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH (2002) Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol 283:G1257–G1263
Souba WW, Klimberg VS, Hautamaki RD, Mendenhall WH, Bova FC, Howard RJ et al (1990) Oral glutamine reduces bacterial translocation following abdominal radiation. J Surg Res 48:1–5
Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G (1995) Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol 268:G374–G379
Takayama C, Mukaizawa F, Fujita T, Ogawara K, Higaki K, Kimura T (2009) Amino acids suppress apoptosis induced by sodium laurate, an absorption enhancer. J Pharm Sci 98:4629–4638
Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141:769–776
van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M et al (2012) Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat Cell Biol 14:829–837
Veldhoen M, Brucklacher-Waldert V (2012) Dietary influences on intestinal immunity. Nat Rev Immunol 12:696–708
Wang J, Chen L, Li P, Li X, Zhou H, Wang F et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wijtten PJ, van der Meulen J, Verstegen MW (2011) Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr 105:967–981
Wischmeyer PE (2002) Glutamine and heat shock protein expression. Nutrition 18:225–228
Wu G (1996) Effects of concanavalin A and phorbol myristate acetate on glutamine metabolism and proliferation of porcine intestinal intraepithelial lymphocytes. Comp Biochem Physiol A Physiol 114:363–368
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paraddigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Knabe DA, Yan W, Flynn NE (1995) Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am J Physiol 268:R334–R342
Wu G, Meier SA, Knabe DA (1996) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE et al (2011) Triennial Growth Symposium: important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C et al (2013) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xi P, Jiang Z, Zheng C, Lin Y, Wu G (2011) Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci (Landmark Ed) 16:578–597
Xi P, Jiang Z, Dai Z, Li X, Yao K, Zheng C et al (2012) Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem 23:1012–1017
Yang Y, Sun F, Zhang C et al (2013) Hypoxia promotes cell proliferation by modulating E2F1 in chicken pulmonary arterial smooth muscle cells. J Anim Sci Biotechnol 4:28
Yao K, Yin Y, Li X, Xi P, Wang J, Lei J et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:2491–2500
Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL et al (2005) Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+ -challenged weaned pigs. J Anim Sci 83:634–643
Zhang SH, Qiao SY, Ren M et al (2013) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45:1191–1205
Ziegler TR (1994) Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. JPEN J Parenter Enteral Nutr 18:84–86
Acknowledgments
This work was supported by National Key Basic Research Program (2013CB127302), the Natural Science Foundation of China (31172217, 31272450, 31301979, and 31272451), the Chinese Universities Scientific Fund (2013RC002), the Program for New Century Excellent Talents in University (NCET-12-0522), and the Program for Beijing Municipal Excellent Talents, and Texas A&M AgriLife Research (H-8200).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Wu, G., Zhou, Z. et al. Glutamine and intestinal barrier function. Amino Acids 47, 2143–2154 (2015). https://doi.org/10.1007/s00726-014-1773-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1773-4