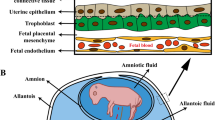
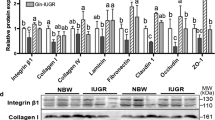
Abstract
Intrauterine growth restriction (IUGR) is one of the most common concerns in human obstetrics and domestic animal production. It is usually caused by placental insufficiency, which decreases fetal uptake of nutrients (especially amino acids) from the placenta. Amino acids are not only building blocks for protein but also key regulators of metabolic pathways in fetoplacental development. The enhanced demands of amino acids by the developing conceptus must be met via active transport systems across the placenta as normal pregnancy advances. Growing evidence indicates that IUGR is associated with a reduction in placental amino acid transport capacity and metabolic pathways within the embryonic/fetal development. The positive relationships between amino acid concentrations in circulating maternal blood and placental amino acid transport into fetus encourage designing new therapies to prevent or treat IUGR by enhancing amino acid availability in maternal diets or maternal circulation. Despite the positive effects of available dietary interventions, nutritional therapy for IUGR is still in its infancy. Based on understanding of the underlying mechanisms whereby amino acids promote fetal growth and of their dietary requirements by IUGR, supplementation with functional amino acids (e.g., arginine and glutamine) hold great promise for preventing fetal growth restriction and improving health and growth of IUGR offspring.
Similar content being viewed by others
Abbreviations
- AGA:
-
Appropriate for gestational age
- BCAA:
-
Branched-chain amino acids
- eNOS:
-
Endothelial nitric oxide synthase
- IUGR:
-
Intrauterine growth restriction
- mTOR:
-
Mammalian target of rapamycin
- NBW:
-
Normal birth weight
- NO:
-
Nitric oxide
- NRC:
-
National research council
- ODC:
-
Ornithine decarboxylase
- SGA:
-
Small for gestational age
References
Alexandre-Gouabau MC, Courant F, Le Gall G et al (2011) Offspring metabolomic response to maternal protein restriction in a rat model of intrauterine growth restriction (IUGR). J Proteome Res 10:3292–3302
American College of Obstetricians and Gynecologists (2001) Intrauterine growth restriction. Clinical management guidelines for obstetrician-gynecologists. Int J Gynaecol Obstet 72:85–96
Avagliano L, Garo C, Marconi AM (2012) Placental amino acids transport in intrauterine growth restriction. J Pregnancy 2012:972562
Battaglia FC, Meschia G (1988) Fetal nutrition. Annu Rev Nutr 8:43–61
Bazer FW, Spencer TE, Johnson GA et al (2009) Comparative aspects of implantation. Reproduction 138:195–209
Bazer FW, Kim J, Ka H et al (2012) Select nutrients in the uterine lumen of sheep and pigs affect conceptus development. J Reprod Dev 58:180–188
Belkacemi L, Nelson DM, Desai M et al (2010) Maternal undernutrition influences placental-fetal development. Biol Reprod 83:325–331
Bérard J, Bee G (2010) Effects of dietary l-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal 4:1680–1687
Bhasin KK, van Nas A, Martin LJ et al (2009) Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 58(3):559–566
Blickstein I (2005) Growth aberration in multiple pregnancy. Obstet Gyn Clin North Am 32:39–54
Blickstein I, Kalish RB (2003) Birthweight discordance in multiple pregnancy. Twin Res 6:526–531
Brown LD, Green AS, Limesand SW et al (2011) Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 3:428–444
Brown LD, Rozance PJ, Thorn SR et al (2012) Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 303:E352–E364
Bryk J, Ochoa JB, Correia MI et al (2008) Effect of citrulline and glutamine on nitric oxide production in RAW 264.7 cells in an arginine-depleted environment. J Parenter Enteral Nutr 32:377–383
Cariappa R, Heath-Monnig E, Smith CH (2003) Isoforms of amino acid transporters in placental syncytiotrophoblast: plasma membrane localization and potential role in maternal/fetal transport. Placenta 24:713–726
Casanello P, Sobrevia L (2002) Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y+/hCAT-1 and y+/hCAT-2B and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ Res 91(2):127–134
Cecconi D, Lonardoni F, Favretto D et al (2011) Changes in amniotic fluid and umbilical cord serum proteomic profiles of foetuses with intrauterine growth retardation. Electrophoresis 32:3630–3637
Cetin I, Fennessey PV, Quick AN et al (1991) Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am J Physiol 260:E371–E378
Cetin I, Ronzoni S, Marconi AM et al (1996) Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol 174:1575–1583
Christensen HN (1990) Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 70:43–77
Coan PM, Vaughan OR, Sekita Y et al (2010) Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 588:527–538
Cosmi E, Visentin S, Favretto D et al (2013) Selective intrauterine growth restriction in monochorionic twin pregnancies: markers of endothelial damage and metabolomic profile. Twin Res Hum Genet 1–11
Curi R, Lagranha CJ, Doi SQ (2005) Molecular mechanisms of glutamine action. J Cell Physiol 204:392–401
Dai ZL, Wu ZL, Yang Y et al (2013) Nitric oxide and energy metabolism in mammals. BioFactors 39:383–391
Davis TA, Fiorotto ML, Burrin DG et al (1997) Intrauterine growth restriction does not alter response of protein synthesis to feeding in newborn pigs. Am J Physiol 272:E877–E884
Davis TA, Fiorotto ML, Burrin DG et al (2002) Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282:E880–E890
de Boo HA, van Zijl PL, Lafeber HN et al (2007) Urea production and arginine metabolism are reduced in the growth restricted ovine foetus. Animal 1:699–707
de Boo HA, Eremia SC, Bloomfield FH et al (2008) Treatment of intrauterine growth restriction with maternal growth hormone supplementation in sheep. Am J Obstet Gynecol 199(559):e551–e559
Del Curto H, Wu H, Satterfield MC (2013) Nutrition and reproduction: links to epigenetics and metabolic syndrome in offspring. Curr Opin Clin Nutr Metab Care 16:385–391
Economides DL, Nicolaides KH, Gahl WA et al (1989) Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161:1219–1227
Faraci M, Renda E, Monte S et al (2011) Fetal growth restriction: current perspectives. J Prenat Med 5:31–33
Favretto D, Cosmi E, Ragazzi E et al (2012) Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem 402:1109–1121
Freetly HC, Leymaster KA (2004) Relationship between litter birth weight and litter size in six breeds of sheep. J Anim Sci 82:612–618
Froen JF, Gardosi JO, Thurmann A et al (2004) Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 83:801–807
Furness DL, Fenech MF, Khong YT et al (2008) One-carbon metabolism enzyme polymorphisms and uteroplacental insufficiency. Am J Obstet Gynecol 199(276):e271–e278
Gao H, Wu G, Spencer TE et al (2009a) Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod 80:602–609
Gao H, Wu G, Spencer TE et al (2009b) Select nutrients in the ovine uterine lumen. I. Amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80:86–93
Gao KG, Jiang ZY, Lin YC et al (2012) Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42:2207–2214
Gootwine E, Spencer TE, Bazer FW (2007) Litter-size-dependent intrauterine growth restriction in sheep. Animal 1:547–564
Gwatkin RB (1966) Defined media and development of mammalian eggs in vitro. Ann N Y Acad Sci 139:79–90
Harding JE (2001) The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 30:15–23
Haugen G, Kiserud T, Godfrey K et al (2004) Portal and umbilical venous blood supply to the liver in the human fetus near term. Ultrasound Obstet Gynecol 24:599–605
Hayes KC, Sturman JA (1981) Taurine in metabolism. Annu Rev Nutr 1:401–425
Haynes TE, Li P, Li XL et al (2009) l-Glutamine or L-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
He Q, Ren P, Kong X et al (2011) Intrauterine growth restriction alters the metabonome of the serum and jejunum in piglets. Mol Biosyst 7:2147–2155
Hefler LA, Reyes CA, O’Brien WE et al (2001) Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol Reprod 64:666–673
Helmbrecht GD, Farhat MY, Lochbaum L et al (1996) l-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Am J Obstet Gynecol 175:800–805
Hugentobler SA, Diskin MG, Leese HJ et al (2007) Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol Reprod Dev 74:445–454
Hultman K, Alexanderson C, Manneras L et al (2007) Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol 579:823–833
Ishida M, Hiramatsu Y, Masuyama H et al (2002) Inhibition of placental ornithine decarboxylase by DL-alpha-difluoro-methyl ornithine causes fetal growth restriction in rat. Life Sci 70:1395–1405
Ivorra C, Garcia-Vicent C, Chaves FJ et al (2012) Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J Transl Med 10:142
Jansson T, Powell TL (2007) Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 113:1–13
Jansson T, Scholtbach V, Powell TL (1998) Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res 44:532–537
Jansson N, Pettersson J, Haafiz A et al (2006) Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576:935–946
Jobgen WS, Ford SP, Jobgen SC et al (2008) Baggs ewes adapt to maternal undernutrition and maintain conceptus growth by maintaining fetal plasma concentrations of amino acids. J Anim Sci 86:820–826
Jozwik M, Teng C, Wilkening RB et al (2004) Reciprocal inhibition of umbilical uptake within groups of amino acids. Am J Physiol Endocrinol Metab 286:E376–E383
Kavitha JV, Rosario FJ, Nijland MJ et al (2014) Down-regulation of placental mTOR, insulin/IGF-I signaling and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 28:1294–1305
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95
Kim JY, Burghardt RC, Wu G et al (2011a) Select nutrients in the ovine uterine lumen. VII. Effects of arginine, leucine, glutamine, and glucose on trophectoderm cell signaling, proliferation, and migration. Biol Reprod 84:62–69
Kim JY, Burghardt RC, Wu G et al (2011b) Select nutrients in the ovine uterine lumen. VIII. Arginine stimulates proliferation of ovine trophectoderm cells through MTOR-RPS6 K-RPS6 signaling cascade and synthesis of nitric oxide and polyamines. Biol Reprod 84:70–78
Kim JY, Song GH, Wu G et al (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod 88:113
Knight JW, Bazer FW, Thatcher WW et al (1977) Conceptus development in intact and unilaterally hysterectomized-ovariectomized gilts: interrelations among hormonal status, placental development, fetal fluids and fetal growth. J Anim Sci 44:620–637
Kong X, Tan B, Yin Y et al (2012) l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Kulandavelu S, Whiteley KJ, Qu D et al (2012) Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension 60:231–238
Kulandavelu S, Whiteley KJ, Bainbridge SA et al (2013) Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension 61:259–266
Kwon H, Spencer TE, Bazer FW et al (2003) Developmental changes of amino acids in ovine fetal fluids. Biol Reprod 68:1813–1820
Lassala A, Bazer FW, Cudd TA et al (2009) Intravenous administration of L-citrulline to pregnant ewes is more effective than l-arginine for increasing arginine availability in the fetus. J Nutr 139:660–665
Lassala A, Bazer FW, Cudd TA et al (2010) Parenteral administration of l-arginine prevents fetal growth restriction in undernourished ewes. J Nutr 140:1242–1248
Lassala A, Bazer FW, Cudd TA et al (2011) Parenteral administration of l-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J Nutr 141:849–855
Lei X, Feng C, Liu C et al (2011) Regulation of protein expression by l-arginine in endothelial cells. Front Biosci S3:655–661
Lei J, Feng DY, Zhang YL et al (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Lewis RM, Brooks S, Crocker IP et al (2013) Review: modelling placental amino acid transfer–from transporters to placental function. Placenta 34(Suppl):S46–S51
Li P, Knabe DA, Kim SW et al (2009) Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Li X, Bazer FW, Johnson GA et al (2010) Dietary supplementation with 0.8% l-arginine between days 0 and 25 of gestation reduces litter size in gilts. J Nutr 140:1111–1116
Li XL, Rezaei R, Li P et al (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li XL, Bazer FW, Johnson GA et al (2014) Dietary supplementation with l-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids. doi:10.1007/s00726-013-1626-6
Lin G, Liu C, Wang T et al (2011) Biomarkers for optimal requirements of amino acids by animals and humans. Front Biosci (Schol Ed) 3:1298–1307
Lin G, Liu C, Feng C et al (2012) Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J Nutr 142:990–998
Liu J, Liu L, Chen H (2011) Antenatal taurine supplementation for improving brain ultrastructure in fetal rats with intrauterine growth restriction. Neuroscience 181:265–270
Liu C, Lin G, Wang X et al (2013a) Intrauterine growth restriction alters the hepatic proteome in fetal pigs. J Nutr Biochem 24:954–959
Liu J, Liu Y, Wang XF et al (2013b) Antenatal taurine supplementation improves cerebral neurogenesis in fetal rats with intrauterine growth restriction through the PKA-CREB signal pathway. Nutr Neurosci 16:282–287
Locasale JW (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13:572–583
MacLennan NK, James SJ, Melnyk S et al (2004) Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics 18:43–50
Mandruzzato G, Antsaklis A, Botet F et al (2008) Intrauterine restriction (IUGR). J Perinat Med 36:277–281
Manso HE, Filho HC, de Carvalho LE et al (2012) Glutamine and glutamate supplementation raise milk glutamine concentrations in lactating gilts. J Anim Sci Biotechnol 3:2
Mateo RD, Wu G, Bazer FW et al (2007) Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Mateo RD, Wu G, Moon HK et al (2008) Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci 86:827–835
McCoard S, Sales F, Wards N et al (2013) Parenteral administration of twin-bearing ewes with l-arginine enhances the birth weight and brown fat stores in sheep. SpringerPlus 2:684
Moco S, Collino S, Rezzi S et al (2013) Metabolomics perspectives in pediatric research. Pediatr Res 73:570–576
Moores RR Jr, Rietberg CC, Battaglia FC et al (1993) Metabolism and transport of maternal serine by the ovine placenta: glycine production and absence of serine transport into the fetus. Pediatr Res 33:590–594
Nathanielsz PW (2006) Decreased placental amino acid transport and intrauterine growth restriction: which is the chicken and which is the egg? J Physiol 576:649
Norberg S, Powell TL, Jansson T (1998) Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res 44:233–238
Novak DA, Beveridge MJ, Malandro M et al (1996) Ontogeny of amino acid transport system A in rat placenta. Placenta 17:643–651
NRC (1998) Nutrient requirements of Swine, 10th revised edn. Natl Acad Press, Washington, DC
NRC (2012) Nutrient requirements of Swine, 11th revised edn. Natl Acad Press, Washington, DC
Padoan A, Rigano S, Ferrazzi E et al (2004) Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol 191:1459–1464
Paolini CL, Teng C, Jozwik M et al (2003) Umbilical threonine uptake during maternal threonine infusion in sheep. Placenta 24:354–360
Parimi PS, Cripe-Mamie C, Kalhan SC (2004) Metabolic responses to protein restriction during pregnancy in rat and translation initiation factors in the mother and fetus. Pediatr Res 56:423–431
Park JH, Stoffers DA, Nicholls RD et al (2008) Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118:2316–2324
Philipps AF, Holzman IR, Teng C et al (1978) Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol 131:881–887
Pomorski M, Zimmer M, Florjanski J et al (2012) Comparative analysis of placental vasculature and placental volume in normal and IUGR pregnancies with the use of three-dimensional Power Doppler. Arch Gynecol Obstet 285:331–337
Raimondi F, Spera AM, Sellitto M et al (2012) Amino acid-based formula as a rescue strategy in feeding very-low-birth-weight infants with intrauterine growth restriction. J Pediatr Gastroenterol Nutr 54:608–612
Ramadoss J, Wu G, Cudd TA (2008) Chronic binge ethanol-mediated acidemia reduces availability of glutamine and related amino acids in maternal plasma of pregnant sheep. Alcohol 42(8):657–666
Ramaekers P, Kemp B, van der Lende T (2006) Progenos in sows increases number of piglets born. J Anim Sci 84(Suppl 1):394 (Abstract)
Reeds PJ (2000) Dispensable and indispensable amino acids for humans. J Nutr 130:1835S–1840S
Regnault TR, de Vrijer B, Battaglia FC (2002) Transport and metabolism of amino acids in placenta. Endocrine 19:23–41
Regnault TR, Friedman JE, Wilkening RB et al (2005) Fetoplacental transport and utilization of amino acids in IUGR–a review. Placenta 26(Suppl A):S52–S62
Ren W, Yin YL, Liu G et al (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Rezaei R, Wang WW, Wu ZL et al (2013) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotech 4:7
Ronzoni S, Marconi AM, Paolini CL et al (2002) The effect of a maternal infusion of amino acids on umbilical uptake in pregnancies complicated by intrauterine growth restriction. Am J Obstet Gynecol 187:741–746
Roos S, Powell TL, Jansson T (2004) Human placental taurine transporter in uncomplicated and IUGR pregnancies: cellular localization, protein expression, and regulation. Am J Physiol Regul Integr Comp Physiol 287:R886–R893
Roos S, Jansson N, Palmberg I et al (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582:449–459
Ross JC, Fennessey PV, Wilkening RB et al (1996) Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol 270:E491–E503
Rozance PJ, Crispo MM, Barry JS et al (2009) Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab 297:E638–E646
Sales F, Pacheco D, Blair H et al (2013) Muscle free amino acid profiles are related to differences in skeletal muscle growth between single and twin ovine fetuses near term. Springerplus 2:483
Satterfield MC, Bazer FW, Spencer TE et al (2010) Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr 140:251–258
Satterfield MC, Dunlap KA, Keisler DH et al (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 43:1593–1603
Satterfield MC, Dunlap KA, Keisler DH et al (2013) Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids 45:489–499
Say L, Gulmezoglu AM, Hofmeyr GJ (2003) Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst Rev (1):CD000148
Schaffer SW, Ito T, Azuma J (2014) Clinical significance of taurine. Amino Acids 46:1–5
Self JT, Spencer TE, Johnson GA et al (2004) Glutamine synthesis in the developing porcine placenta. Biol Reprod 70:1444–1451
Shen SF, Hua CH (2011) Effect of l-arginine on the expression of Bcl-2 and Bax in the placenta of fetal growth restriction. J Matern Fetal Neonatal Med 24:822–826
Shibata E, Hubel CA, Powers RW et al (2008) Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta 29(10):879–882
Sieroszewski P, Suzin J, Karowicz-Bilinska A (2004) Ultrasound evaluation of intrauterine growth restriction therapy by a nitric oxide donor (l-arginine). J Matern Fetal Neonatal Med 15:363–366
Story L, Damodaram MS, Allsop JM et al (2011) Brain metabolism in fetal intrauterine growth restriction: a proton magnetic resonance spectroscopy study. Am J Obstet Gynecol 205(483):e481–e488
Tea I, Le Gall G, Kuster A et al (2012) 1H-NMR-based metabolic profiling of maternal and umbilical cord blood indicates altered materno-foetal nutrient exchange in preterm infants. PLoS One 7:e29947
Teasdale F, Jean-Jacques G (1985) Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from mid-gestation to term. Placenta 6:375–381
Thorn SR, Rozance PJ, Brown LD et al (2011) The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29:225–236
Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A et al (2006) Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci 1092:138–147
van Vliet E, Eixarch E, Illa M et al (2013) Metabolomics reveals metabolic alterations by intrauterine growth restriction in the fetal rabbit brain. PLoS One 8:e64545
Vaughn PR, Lobo C, Battaglia FC et al (1995) Glutamine-glutamate exchange between placenta and fetal liver. Am J Physiol 268:E705–E711
Viana LR, Gomes-Marcondes MC (2013) Leucine-rich diet improves the serum amino acid profile and body composition of fetuses from tumor-bearing pregnant mice. Biol Reprod 88:121
Vosatka RJ, Hassoun PM, Harvey-Wilkes KB (1998) Dietary l-arginine prevents fetal growth restriction in rats. Am J Obstet Gynecol 178:242–246
Wang J, Chen L, Li D et al (2008a) Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 138:60–66
Wang J, Chen L, Li P et al (2008b) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang X, Wu W, Lin G et al (2010) Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction. J Proteome Res 9:924–935
Wang J, Wu Z, Li D et al (2012a) Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal 17:282–301
Wang Y, Zhang L, Zhou G et al (2012b) Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr 108:1371–1381
Wang T, Liu C, Feng C et al (2013a) IUGR alters muscle fiber development and proteome in fetal pigs. Front Biosci 18:598–607
Wang W, Wu Z, Dai Z et al (2013b) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Washburn SE, Sawant OB, Lunde ER et al (2013a) Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids 45:543–554
Washburn SE, Sawant OB, Wu G (2013b) Maternal glutamine supplementation mitigates negative fetal developmental effects from prenatal alcohol exposure. Amino Acids 45:602
Waterland RA, Jirtle RL (2004) Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 20:63–68
Webel SK, Dziuk PJ (1974) Effect of stage of gestation and uterine space on prenatal survival in the pig. J Anim Sci 38:960–963
Winer N, Branger B, Azria E et al (2009) l-Arginine treatment for severe vascular fetal intrauterine growth restriction: a randomized double-bind controlled trial. Clin Nutr 28:243–248
Wootton R, Flecknell PA, Royston JP et al (1983) Intrauterine growth retardation detected in several species by non-normal birthweight distributions. J Reprod Fertil 69:659–663
Wu G (2013) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2014) Dietary requirements of “nutritionally nonessential amino acids” by mammals. Amino Acids Res (Jpn) 7:67–76
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Meininger CJ (2000) Arginine nutrition and cardiovascular function. J Nutr 130:2626–2629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Bazer FW, Tou W (1995) Developmental changes of free amino acid concentrations in fetal fluids of pigs. J Nutr 125:2859–2868
Wu G, Bazer FW, Tuo W et al (1996) Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol Reprod 54:1261–1265
Wu G, Pond WG, Ott T et al (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128(5):894–902
Wu G, Ott TL, Knabe DA et al (1999) Amino acid composition of the fetal pig. J Nutr 129:1031–1038
Wu G, Bazer FW, Cudd TA et al (2004a) Maternal nutrition and fetal development. J Nutr 134:2169–2172
Wu G, Knabe DA, Kim SW (2004b) Arginine nutrition in neonatal pigs. J Nutr 134(10 Suppl):2783S–2790S
Wu G, Bazer FW, Hu J et al (2005) Polyamine synthesis from proline in the developing porcine placenta. Biol Reprod 72:842–850
Wu G, Bazer FW, Wallace JM et al (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Wu G, Bazer FW, Davis TA et al (2007) Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci 112:8–22
Wu G, Bazer FW, Datta S et al (2008) Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids 35:691–702
Wu G, Bazer FW, Burghardt RC et al (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 88(13 Suppl):E195–E204
Wu G, Bazer FW, Johnson GA et al (2011) Triennial growth symposium: important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Imhoff-Kunsch B, Girard AW (2012a) Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinatal Epidemiol 26(Suppl 1):4–26
Wu ZL, Satterfield MC, Bazer FW et al (2012b) Regulation of brown adipose tissue development and white fat reduction by l-arginine. Curr Opin Clin Nutr Metab Care 15:529–538
Wu G, Bazer FW, Johnson GA et al (2013a) Maternal and fetal amino acid metabolism in gestating sows. Soc Reprod Fertil (Suppl) 68:185–198
Wu G, Bazer FW, Satterfield MC et al (2013b) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Wu ZL, Dai ZL et al (2013c) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Dai ZL et al (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Xi PB, Jiang ZY, Zheng CT et al (2011) Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci 16:578–597
Xiao XM, Li LP (2005) l-Arginine treatment for asymmetric fetal growth restriction. Int J Gynaecol Obstet 88:15–18
Yates DT, Macko AR, Nearing M et al (2012) Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J Pregnancy 2012:631038
Zeng X, Wang F, Fan X et al (2008) Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr 138:1421–1425
Zeng X, Huang Z, Mao X et al (2012) N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One 7:e41192
Zheng C, Huang C, Cao Y et al (2009) Branched-chain amino acids reverse the growth of intrauterine growth retardation rats in a malnutrition model. Asian Aust J Anim Sci 22:1495–1503
Zhou WL, Gosch G, Guerra T et al (2014) Amino acid profiles in first trimester amniotic fluids of healthy bovine cloned pregnancies are similar to those of IVF pregnancies, but not nonviable cloned pregnancies. Theriogenology 81:225–229
Acknowledgments
This work was supported by the Natural Science Foundation of China (no. 30810103902, 30972156, 31129006, 31272449, and 31272450), and Texas A&M AgriLife Research (H-8200).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, G., Wang, X., Wu, G. et al. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids 46, 1605–1623 (2014). https://doi.org/10.1007/s00726-014-1725-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1725-z