Abstract
Newsholme’s theory of central fatigue suggests that acute tryptophan depletion should improve endurance exercise capacity in a warm environment by reducing serotonergic activity in the brain. Eight males cycled to volitional exhaustion at 55 % \( \dot{V}{\rm O}_{2} \) peak in 30.1 ± 0.5 °C and 30 ± 7 % relative humidity on two separate occasions, after consuming either an amino acid load to deplete their circulating tryptophan concentration (TD), or a control amino acid load (CON). Blood samples were taken before ingesting the amino acids, before the start of exercise, every 15 min during exercise and at the point of exhaustion. Heart rate (HR), core (Tc) and skin (Tsk) temperatures and ratings of perceived exertion (RPE) and thermal comfort (TC) were also monitored every 10 min during exercise. Plasma tryptophan (P = 0.003) and free tryptophan (P < 0.001) concentrations, and the free tryptophan to branched-chain amino acid ratio (P = 0.004) were all lower on the TD trial than on the CON trial. There was no difference in endurance exercise capacity (TD 99.2 ± 24.4 min as compared to CON 108.4 ± 21.6 min; P = 0.088). There was a tendency for HR (P = 0.053) and Tc (P = 0.069) to be higher on the TD trials. There were no differences for any of the other parameters. Endurance cycling capacity in a warm environment is not improved by acute tryptophan depletion, suggesting tryptophan availability is not a significant factor in the development of fatigue in such situations.
Similar content being viewed by others
Introduction
The cause of fatigue during exercise in a temperate environment can be attributed to depletion of the muscle glycogen content (Ahlborg et al. 1967), but the underlying limitations to performance in a warm environment appear to be more complex. It has been suggested that the central nervous system may play an important role in the fatigue process when body temperature is significantly elevated (Nielsen 1992; Nybo and Nielsen 2001). Prolonged strenuous exercise increases serotonin synthesis and release, leading to suggestions that elevated serotonin concentrations may lead to fatigue and an impaired exercise capacity (Newsholme et al. 1987).
Changes in central serotonergic neurotransmission have been implicated in the development of fatigue due to the involvement of serotonin (5-hydroxytryptamine, 5-HT) in the control of arousal, sleepiness and mood (Jacobs and Azimita 1992). Support for this hypothesis was provided by Wilson and Maughan (1992), who demonstrated that when Paroxetine (a serotonin reuptake inhibitor) was administered before prolonged exhaustive cycling, exercise capacity was significantly impaired relative to a placebo condition. These findings were supported by Struder et al. (1998) providing further evidence for a role of serotonin in the development of fatigue.
Serotonin is synthesised in the body from the essential amino acid precursor tryptophan (Trp). A large proportion of the tryptophan in the circulation is bound to albumin, and only the unbound, or free, fraction is available for uptake into tissues. Once free tryptophan is transported across the blood brain barrier, it is converted to serotonin through two enzymatic reactions. The first of these enzymes, tryptophan hydroxylase, is only around 50 % saturated in normal circumstances, meaning that the availability of the substrate, i.e. free tryptophan, is a primary determinant of serotonin formation (Cooper et al. 2003). An elevated plasma Trp concentration has been shown to increase brain concentrations of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA), the principal 5-HT metabolite, in rats (Chaouloff 1989). Brain 5-HT concentration in turn regulates the rate of neurotransmitter release (Schaechter and Wurtman 1990). In accordance with Newsholme’s theory of central fatigue, supplementing tryptophan should elicit a reduction in exercise capacity by increasing Trp uptake into serotonergic neurones and hence increasing 5-HT synthesis. This response has been demonstrated in horses (Farris et al. 1998) and rats (Soares et al. 2007), but mixed results have been reported in human studies (Alves et al. 1995; Stensrud et al. 1992; Van Hall et al. 1995).
Although evidence suggests that 5-HT probably does not play a significant role in the fatigue process in humans, this has mostly been investigated through the supplementation of branched-chain amino acids (BCAA) to change the Trp/BCAA ratio. The present study employs a protocol to manipulate this ratio through the reduction in Trp concentration, as well as an elevation in the circulating concentration of the competing large neutral amino acids (LNAA). This dual approach should further reduce cerebral Trp availability and limit the production of serotonin during exercise.
Acute removal of dietary Trp intake reduces plasma Trp concentration by only about 20 %, and thus has few behavioural consequences (Bell et al. 2001). By combining a low Trp diet with the acute ingestion of a Trp-free amino acid load, it is possible to further reduce plasma free and total Trp concentrations by up to 80 % within 5–7 h (Delgardo et al. 1990). The ingestion of an amino acid load during acute Trp depletion stimulates protein synthesis in the liver (Harper et al. 1970). This process requires Trp which, due to its omission from the ingested amino acid load, is drawn from the circulation. The amino acid load also increases the circulating large neutral amino acid concentration and, due to the increased competition for the same active transport mechanism, a further reduction in the amount of tryptophan passing across the blood–brain barrier occurs (Pardridge 1983).
A reduction in Trp concentration should therefore reduce the synthesis and release of serotonin in the CNS. Williams et al. (1999) showed that acute Trp depletion results in reductions in cerebrospinal fluid Trp and 5-HIAA concentrations. Furthermore, using positron emission tomography (PET), Williams et al. (2002) have shown that acute Trp depletion leads to regional decreases in 5-HT1A receptor occupancy. Indeed, acute Trp depletion has been used extensively in clinical studies into disorders thought to be related to altered serotonergic activity, such as depression, anxiety, aggression, sleep patterns, memory, eating disorders (Bell et al. 2001) as well as cognitive function (Gallagher et al. 2003). Several such investigations have shown alterations in behavioural outcomes following acute Trp depletion, including relapse in recovering major depressive disorder patients (Neumeister et al. 2004) impaired long-term memory formation (Schmitt et al. 2000) and increased levels of aggression (McCloskey et al. 2009).
Based on the available evidence, acute Trp depletion, resulting in a significant reduction in brain serotonin concentration, might be expected to improve endurance exercise capacity. There is not much information on the effect of Trp depletion on the performance of endurance exercise, and, despite the evidence rejecting Newsholme’s theory of central fatigue, Stepto et al. (2011) recently showed that administration of a high dose of LNAA 3 h prior to intermittent high-intensity exercise improved motor skill and agility performance in football players. Therefore, the aim of this investigation was to examine the effects of an amino acid load intended to deplete Trp on the response to endurance exercise in a warm environment.
Methods
Participants and ethical approval
Eight healthy, non-acclimated, recreationally active males (mean ± SD: age 24 ± 3 years; height 1.84 ± 0.07 m; mass 84.4 ± 9.9 kg; \( \dot{V}{\rm O}_{2} \) peak 52.8 ± 7.1 ml/kg/min) were recruited to participate in this study. The protocol received approval from the Loughborough University Ethical Advisory Committee and participants gave written informed consent before participation.
Protocol
Participants completed a preliminary discontinuous, incremental cycle ergometer (Gould Corival 300, Holland) test to volitional exhaustion to determine \( \dot{V}{\rm O}_{2} \) peak and the power output required to elicit 55 % \( \dot{V}{\rm O}_{2} \) peak. All subsequent trials were separated by at least 1 week and occurred at the same time of day. The first two trials were for familiarisation purposes, to ensure the subjects were accustomed to the procedures employed and to minimise any potential learning effect. The third and fourth trials were the main experimental trials. All of these trials followed the same protocol, with only the amino acid content of the drink varying. The study was conducted using a randomised, double-blinded, cross-over design.
In the 48 h prior to the first familiarisation trial, participants were asked to record their dietary intake and physical activity patterns. During the 24 h prior to the first familiarisation trial, participants were asked to consume a low tryptophan diet (a list of suitable foods was provided), and to avoid alcohol and strenuous exercise. This diet and activity pattern was then replicated before each of the subsequent trials.
Each trial involved two visits to the laboratory. Participants were asked to attend the laboratory between 5 and 7 p.m. the day before the exercise trial, having consumed nothing but plain water for the previous 4 h. A resting blood sample (7 ml) was taken and participants were then given an amino acid load to take away and consume at 11.30 p.m. that night. The amino acid load consisted of either 104.4 g of amino acids (SHS International ltd, UK) including tryptophan [CON: alanine (5.5 g), arginine (4.9 g), cysteine (2.7 g), glycine (3.2 g), histidine (3.2 g), isoleucine (8 g), leucine (13.5 g), lysine monohydrochloride (11 g), methionine (3 g), phenylalanine (5.7 g), proline (12.2 g), serine (6.9 g), threonine (6.5 g), tyrosine (6.9 g), valine (8.9 g) and tryptophan (2.3 g)], or the same amino acid load excluding tryptophan (TD). To facilitate ingestion of the amino acids, they were divided into a drink and capsules. The gelatine capsules (TAAB, UK) contained isoleucine, tyrosine and methionine, while the drink contained the remaining amino acids along with 8.25 g of intense pineapple flavouring (SHS International ltd, UK) in 350 ml of tap water. This protocol has been previously demonstrated to reduce circulating TRP concentrations (Gallagher et al. 2003) and lower cerebrospinal fluid tryptophan and 5-HIAA concentrations (Williams et al. 1999).
Participants then reported to the laboratory at 5 a.m. the following morning after an overnight fast other than the ingestion of 500 ml of water at 4.30 a.m. Upon arrival, participants emptied their bladder and a sample of the urine was retained and analysed for osmolality by freezing point depression (Osmomat 030 cryoscopic osmometer, Gonotec, Berlin, Germany). Post-void, nude body mass was noted and they then positioned a rectal thermometer 10 cm beyond the anal sphincter to allow the measurement of core body temperature (YSI 400 series, OH, USA). Skin thermistors were positioned on the chest, triceps, thigh and calf (YSI UK Ltd, Hampshire, UK) to enable the calculation of weighted mean skin temperature (Ramanathan 1964). Body heat content was calculated as previously described by Burton (1935) and the rate of rise in core temperature was calculated as the difference between core temperature pre-exercise and at exhaustion, divided by the exercise time to exhaustion. A heart rate telemetry band (Polar Electro Oy, Kempele, Finland) was also worn.
Participants were then seated in a comfortable environment for 15 min and one arm was immersed in warm (41 °C) water before an indwelling cannula was inserted into a superficial forearm vein and an arterialised baseline blood sample (7 ml) was drawn. The cannula was kept patent between samples by flushing with a small volume of heparinised saline. Baseline measures of heart rate and core and skin temperatures were taken before participants moved to a warm environment (30.1 ± 0.5 °C, 30 ± 7 % relative humidity) and exercised on a cycle ergometer at a workload corresponding to 55 % \( \dot{V}{\rm O}_{2} \) peak until volitional exhaustion, defined as an inability to maintain a pedal cadence >50 rpm despite verbal encouragement.
Blood samples (7 ml) were taken at 15 min intervals during exercise and participants consumed 100 ml of plain water immediately after each sample. Heart rate, core temperature, skin temperature and ratings of perceived exertion (Borg 1982) and thermal comfort (21-point scale ranging from ‘unbearable cold’ to ‘unbearable heat’) were recorded every 10 min during exercise; expired gas samples were collected every 30 min. A final blood sample and further temperature readings were taken at the point of exhaustion. Participants then moved back into a comfortable environment and their recovery was monitored. Final recordings of heart rate, core temperature and skin temperature were made 15 min after exercise before the removal of all instruments and the cannula. Participants were reweighed before showering and being provided with food and drinks.
Blood handling and analysis
All 7 ml blood samples were collected into dry syringes. Of this, 5 ml was dispensed into a heparinised tube which was centrifuged for 15 min at 3,000 rpm. A 500 μl aliquot of the heparinised plasma was further centrifuged at 1,500 rpm for 120 min in an ultra-filtrate tube with a cellulose tri-acetate membrane to retain the molecules with a molecular weight of >12,000 (VectaSpin 12K, Whatman International Ltd, Kent, UK). The resulting ultra-filtrate and the remaining heparinised plasma were stored at −80 °C for the analysis of free Trp and large neutral amino acid concentrations (Henderson et al. 2000) by high-performance liquid chromatography (Shimadzu, Kyoto, Japan) using a 4.6 × 75 mm and 3.5 μm column with pre-column derivatisation by ortho phthalaldehyde (Agilent Technologies, Cheshire, UK). A 1 ml aliquot of blood was dispensed into an EDTA tube which was centrifuged for 15 min at 3,000 rpm and the resulting plasma was frozen at −80 °C to be used in the analysis of free-fatty acid concentration (Roche Diagnostics, Mannheim, Germany). The final 1 ml of blood was dispensed into an EDTA tube from which two 100 μl aliquots were removed and dispensed into two 1 ml aliquots of icecold 0.3 N PCA. These were then centrifuged for 1 min and the resulting supernatant was used for the analysis of blood glucose concentration (GOD/PAP method, Randox, UK). The remaining EDTA-treated blood was used for the determination of haemoglobin concentration (cyanmethemoglobin method) and haematocrit values (microcentrifugation method): these values were used to calculate changes in plasma volume over time, using the pre-exercise sample as a baseline (Dill and Costill 1974). All analyses were completed in duplicate, with the exception of haematocrit which was measured in triplicate, and amino acids which were measured singularly.
Statistical analysis
Data are presented as mean ± standard deviation unless otherwise stated. With a sample size of n = 8 and α value of 10 %, power was calculated to be 80 % using G*Power v3.1.5 (Heinrich Heine University, Düsseldorf, Germany). Data were tested for normality of distribution using the Shapiro–Wilk test and were then investigated for skewness and kurtosis. Where appropriate, statistical analysis was done by either a two-way repeated measures ANOVA with a Bonferroni’s correction or a paired sample t test. Statistical analysis was carried out using SPSS v14.0, and a significance was set at P < 0.05.
Results
Plasma amino acid concentrations
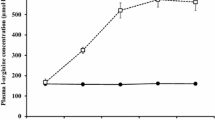
Figure 1a shows plasma Trp and free Trp concentrations at the time points sampled. Trp (P = 0.003) and free Trp (P < 0.001) concentrations are lower on the TD trials than on the CON trials. Figure 1b shows that the ratio of free Trp to BCAA was lower on the TD trials than on the CON trials (P = 0.004). There was also a significant effect of time on the ratio (P = 0.048), which decreased after consumption of the amino acid load and then increased during the exercise phase.
Plasma concentrations of the BCAA and other LNAA are shown in Table 1. No participant reported problems with the ingestion of the amino acid load, and the plasma amino acid data suggest all participants were compliant. Other than leucine, all demonstrated significant changes over time (P < 0.05), with the majority rising either after drinking or during the exercise; none was different between the trials.
Time to exhaustion
There was no effect of trial order on time to exhaustion (trial 1: 102.0 ± 23.4 min, trial 2: 105.6 ± 23.6 min; P = 0.540). The coefficient of variation between the second familiarisation and the CON trial was 8.5 %. There was no effect of acute Trp depletion on exercise capacity (TD 99.2 ± 24.4 min and CON 108.4 ± 21.6 min; P = 0.088). When the data are expressed as a percentage change from the second familiarisation trial, there was still no significant effect of acute Trp depletion (TD 95.5 ± 7.4 % and CON 105.5 ± 10.7 %; P = 0.066).
Thermoregulatory measures
Core temperature increased during the exercise trial (P < 0.001) from resting values of 37.0 ± 0.1 to 38.3 ± 0.3 °C at the point of exhaustion on the TD trial and from 36.9 ± 0.2 to 38.2 ± 0.2 °C on the CON trial (Fig. 2). There was no statistical difference in core temperature between trials (P = 0.069), and there was no difference in the rate of rise of core temperature (P = 0.464).
As with core temperature, skin temperature increased during the exercise test (P < 0.001), but there was no difference between trials (P = 0.597). Skin temperature increased from resting values of 32.5 ± 0.9 to 35.0 ± 0.8 °C at the point of exhaustion on the TD trial, and from 32.3 ± 0.7 to 35.2 ± 0.9 °C on the CON trial. Estimated body heat content also followed a similar pattern to both core and skin temperatures, with no difference between the trials (P = 0.960), but a significant increase during the exercise bout to the point of exhaustion (P < 0.001).
Hydration, cardiovascular and metabolic measures
There was no difference in pre-exercise hydration status, as assessed by urine osmolality, between the two trials (P = 0.427). Body mass loss during exercise was not different between trials (TD 1.43 ± 0.40 kg, CON 1.53 ± 0.32 kg; P = 0.148). There was no effect of time (P = 0.172, P = 0.622) or trial (P = 0.881, P = 0.264) on estimated energy expenditure or the respiratory exchange ratio. There was no effect of time (P = 0.840, P = 0.471) or trial (P = 0.636, P = 0.667) on the contributions of either carbohydrate or fat to oxidative metabolism during exercise.
Rating of perceived exertion increased during exercise (P < 0.001) as did rating of thermal comfort (P < 0.001), but neither were affected by the different trials (P = 0.195; P = 0.422; Table 2). Heart rate was not different between trials (P = 0.053), and increased over the duration of the exercise (P < 0.001) to peak at the point of exhaustion (165 ± 11 and 161 ± 14 beats/min on the TD and the CON trials, respectively, Fig. 3).
Plasma volume changed over time (P < 0.001), but was not different between the trials (P = 0.211). It increased initially between the pre-supplementation sample and the pre-exercise sample, but it then fell during exercise reaching a minimum at the point of exhaustion. Blood glucose concentration also fluctuated over time (P = 0.007), gradually increasing from the pre-exercise sample (TD 5.14 ± 0.41 mmol/L; CON 5.00 ± 0.44 mmol/L) to the point of exhaustion (TD 5.42 ± 0.35 mmol/L; CON 5.25 ± 0.28 mmol/L), although again, there was no difference between the trials (P = 0.666). Free-fatty acid concentrations changed significantly over time (P < 0.001), increasing from pre-exercise (TD 0.34 ± 0.13; CON 0.37 ± 0.14) to exhaustion (TD 1.06 ± 0.34 mmol/L; CON 1.04 ± 0.32 mmol/L), but there was no effect of trial (P = 0.695).
Discussion
The present investigation shows that acute Trp depletion does not improve endurance exercise capacity in a warm environment. TD resulted in a greater than twofold fall in circulating Trp concentrations, consequently reducing the plasma concentration ratio of f-Trp to BCAA by 79 %. With this in mind, these data do not appear to support Newsholme’s theory of central fatigue, and suggest that the availability of tryptophan as a metabolic precursor for serotonin synthesis is not a significant factor in the development of fatigue during prolonged exercise. This finding is further supported by the data of other studies that have attempted to manipulate Trp availability to the brain during exercise, albeit through the provision of BCAA supplements (Watson 2008) rather than through Trp depletion. Indeed, rather than showing an improvement in exercise capacity, our data show a tendency towards an impairment in endurance exercise capacity in the heat with TD as compared to CON (99.2 ± 24.4 and 108.4 ± 21.6 min). We also show a trend for elevated HR (P = 0.053) and Tc (P = 0.069) on the TD trial. Serotonin is known to be involved in the regulation of heart rate (Jordan 2005) and core temperature (Cooper et al. 2003) suggesting that acute Trp depletion may have had some influence on serotonin availability, but it may be that some other effect influenced these parameters.
Ramage and Fozard (1987) administered a serotonin receptor (5-HT1A) agonist to anaesthetised cats and showed that this evoked a vagally mediated fall in the heart rate. In a more recent study, Van der Veen et al. (2008) found that, in a response test designed by Miltner et al. (1997) which provides almost instantaneous positive or negative feedback to participants on their ability to estimate a 1 s interval, heart rate slowed when participants received negative feedback. However, when participants had undergone acute Trp depletion that response was reduced. Furthermore, baroreflex gain was attenuated in rats that had undergone 5-HT depletion through the administration of para-chlorophenylalanine, as compared to untreated controls (Kellett et al. 2005). These results imply that phasic heart rate responses are sensitive to lowered 5-HT. It is possible, therefore, that the administration of an amino acid load designed to deplete the 5-HT precursor Trp, may lead to a relative increase in heart rate, as shown in the present study. It should also be considered that 5-HT is known to have vasoactive properties (Yildiz et al. 1998) and therefore, if a reduction in 5-HT synthesis and release led to vasodilation, heart rate would increase to maintain cardiac output. However, other studies that have used nutritional (Mittleman et al. 1998; Van Hall et al. 1995; Watson et al. 2004) and pharmacological (Wilson and Maughan 1992) interventions in an attempt to manipulate serotonergic function did not see alterations in heart rate.
Because serotonergic and catecholaminergic projections innervate areas of the hypothalamus, changes in the activity of these neurons have been implicated in the control of body temperature during exercise (Hasegawa et al. 2008). If acute Trp depletion causes a reduction in 5-HT production, then this may lead to an increased rectal temperature. Administration of a 5-HT receptor (5-HT2C) antagonist (Pizotifen) to resting, healthy, human participants caused an increase in core temperature (Strachan et al. 2005) a finding that supports the proposed theory that reducing 5-HT concentration through acute Trp depletion could result in an elevated core temperature. However, as with heart rate, BCAA studies have not shown alterations in core temperature (Mittleman et al. 1998; Watson et al. 2004).
Watson et al. (2005) demonstrated that a dopamine/noradrenaline reuptake inhibitor improved exercise time trial performance in the heat compared to a placebo. In the same way that acute Trp depletion can be used to reduce the brain concentration of Trp, acute depletion of tyrosine and phenylalanine has been shown to reduce the brain concentration of dopamine and noradrenaline (McTavish et al. 1999; Palmour et al. 1998) influencing participants’ mood (Leyton et al. 2000). These data, taken in combination, suggest that exercise performance may be impaired by the reduction in brain dopamine/noradrenaline through acute tyrosine and phenylalanine depletion. However, caution should be used when attempting to prove the metabolic mechanisms behind alterations in performance using methodologies designed to impair performance, rather than improve it.
It is important to note that the core temperatures seen at the point of exhaustion in these trials (TD: 38.3 ± 0.3 °C; CON: 38.2 ± 0.2 °C) were low in comparison to those reported in the wider literature on prolonged exercise in the heat. A critical core temperature of 39.7 °C has been proposed, above which exercise cannot be sustained (Nielsen et al. 1993). Although this hypothesis is attractive, many individuals have been reported to maintain performance at core temperatures beyond 40 °C. In addition, there are many reports of exhaustion during prolonged exercise in the heat occurring at core temperatures lower than this proposed ‘critical’ level [38.8 ± 0.1 °C Bridge et al. (2003); 38.8 ± 0.3 °C Cheung and McLellan (1998); 38.7 ± 0.9 °C Hobson et al. (2009); 38.9 ± 0.5 °C Watson et al. (2004)]. Exercise in the heat results in a narrow skin temperature to core temperature gradient, producing a high skin blood flow requirement in an effort to maximise evaporative heat losses. High skin temperatures (>35 °C), and not high core temperature, has been proposed as a primary factor impairing aerobic exercise performance under these conditions (Cheuvront et al. 2010). Although the significance of core temperature for performance during prolonged dynamic exercise has been questioned in recent years, several studies do report a progressive inhibition of motor activation when temperature is elevated above normothermic levels (Morrison et al. 2004; Nybo and Nielsen 2001). Taken together, it appears that interplay between core, brain and skin temperature most likely regulates fatigue during prolonged, dynamic exercise in hot conditions, as highlighted by recent studies (Cheuvront et al. 2010; Maughan et al. 2012).
An increase in brain Trp uptake during prolonged exercise is suggested to contribute to an elevation in central 5-HT activity (Blomstrand et al. 1989); thus, increasing the perception of effort during exercise (Blomstrand et al. 1997). In the present study, acute Trp depletion did not cause a concurrent lowering of perceived exertion during the exercise (TD 18 ± 2, CON 16 ± 1; P = 0.195). Acute Trp depletion has, however, been shown to increase aggression in animals (Bell et al. 2001) and in humans (Bjork et al. 1999) and, therefore, may be more likely to improve exercise test results in directly competitive environments, where aggression is more likely to play a role in overall performance.
In humans, the response to Trp depletion in the cerebrospinal fluid is delayed, with peak depletion occurring 7–10 h after consuming an amino acid load (Williams et al. 1999) as compared to the maximum level of plasma Trp depletion, which occurs 5–7 h after ingestion of an amino acid load (Delgardo et al. 1990). In the present study, participants started exercise approximately 6 h after consuming the amino acid load, which should have corresponded with the peak time for the depletion of plasma tryptophan (Fig. 1a). For all participants, the point of exhaustion was reached 7–9 h after consuming the amino acid load, which should have corresponded with the peak time of tryptophan depletion in the cerebrospinal fluid.
Conclusion
Contradictory to the hypothesis that Trp depletion would improve endurance exercise capacity, this study has shown that there was no difference in exercise capacity between Trp depletion and a control trial. This finding adds further weight to the theory that the availability of Trp as a precursor to 5-HT production is not a significant factor in the development of fatigue during prolonged exercise in a warm environment.
References
Ahlborg BG, Bergstrom J, Brohult J, Ekelund LG, Hultamn E (1967) Human muscle glycogen content and capacity for prolonged exercise after different diets. Foersvarsmedicin 3:85–99
Alves MN, Ferrari-Auarek WM, Pinto KM, Sa KR, Viveiros JP, Pereira HA, Ribeiro AM, Rodrigues LO (1995) Effects of caffeine and tryptophan on rectal temperature, metabolism, total exercise time, rate of perceived exertion and heart rate. Braz J Med Biol Res 28:705–709
Bell C, Abrams J, Nutt D (2001) Tryptophan depletion and its implications for psychiatry. Brit J Psychiat 178:399–405
Bjork JM, Dougherty DM, Moeller FG, Cherek DR, Swann AC (1999) The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food restricted control. Psychopharmacology 142:24–30
Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA (1989) Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions of the rat. Acta Physiol Scand 136:473–481
Blomstrand E, Hassmen P, Ek S, Ekblom B, Newsholme EA (1997) Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiol Scand 159:41–49
Borg GAV (1982) Psychophysical bases of perceived exertion. Med Sci Sport Exerc 14:377–381
Bridge MW, Weller AS, Rayson M, Jones DA (2003) Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol 89:451–459
Burton AC (1935) Human calorimetry II: the average temperature of tissues of the body. J Nutr 9:261–280
Chaouloff F (1989) Physical exercise and brain monoamines: a review. Acta Physiol Scand 137:1–13
Cheung SS, McLellan TM (1998) Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol 84:1731–1739
Cheuvront SN, Kenefick RW, Montain SJ, Sawka MN (2010) Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol 109:1989–1995
Cooper JR, Bloom FE, Roth RH (2003) The biochemical basis of neuropharmacology. Oxford University Press, NY
Delgardo PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR (1990) Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 47:411–418
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes in blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Farris JW, Hinchcliff KW, McKeever KH, Lamb DR, Thompson DL (1998) Effect of tryptophan and of glucose on exercise capacity of horses. J Appl Physiol 85:807–816
Gallagher P, Massey AE, Young AH, McAllister-Williams RH (2003) Effects of acute tryptophan depletion on executive function in healthy male volunteers. BMC Psychiatry 3:10–19
Harper AE, Benevenga NJ, Wohlhueter RM (1970) Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev 50:428–548
Hasegawa H, Piacentini MF, Sarre S, Michotte Y, Ishiwata T, Meeusen R (2008) Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol 586:141–149
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, accurate, sensitive and reproducible HPLC analysis of amino acids. Agilent Technologies
Hobson RM, Clapp EL, Watson P, Maughan RJ (2009) Exercise capacity in the heat is greater in the morning than in the evening in man. Med Sci Sport Exerc 41:174–180
Jacobs BL, Azimita EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–229
Jordan D (2005) Vagal control of the heart: central serotonergic (5-TH) mechanisms. Exp Physiol 90:175–181
Kellett DO, Stanford SC, Machado BH, Jordan D, Ramage AG (2005) Effect of 5-HT depletion on cardiovascular vagal reflex sensitivity in awake and anesthetized rats. Brain Res 1054:61–72
Leyton M, Young SN, Pihl RO, Etezadi S, Lauze C, Blier P, Baker GB, Benkelfat C (2000) Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacol 22:52–63
Maughan RJ, Otani H, Watson P (2012) The influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 112:2313–2321
McCloskey MS, Ben-Zeev D, Lee R, Berman ME, Caccaro EF (2009) Acute tryptophan depletion and self-injurious behavior in aggressive patients and healthy volunteers. Psychopharmacology 203:53–61
McTavish SFB, Cowen PJ, Sharp T (1999) Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology 141:182–188
Miltner WHR, Braun CH, Coles MGH (1997) Event-related brain potentials following incorrect feedback in a time estimation task: evidence for a ‘generic’ neural system for error detection. J Cog Neurosci 9:788–798
Mittleman KD, Ricci MR, Bailey SP (1998) Branched-chain amino acids prolong exercise during heat stress in men and women. Med Sci Sport Exer 30:83–91
Morrison S, Sleivert GG, Cheung SS (2004) Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol 91:729–736
Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC (2004) Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 61:765–773
Newsholme EA, Acworth IN, Blomstrand E (1987) Amino acids, brain neurotransmitters and a functional link between muscle and brain that is important in sustained exercise. In: Benzi G (ed) Advances in myochemistry. John Libbey, London, pp 127–133
Nielsen B (1992) Heat stress causes fatigue. Med Sport Sci 34:207–217
Nielsen B, Hales JRS, Strange S, Christensen NJ, Warberg J, Saltin B (1993) Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460:467–485
Nybo L, Nielsen B (2001) Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91:1055–1060
Palmour RM, Ervin FR, Baker GB, Young SN (1998) An amino acid mixture deficient in phenylalanine and tyrosine reduces cerebrospinal fluid catecholamine metabolites and alcohol consumption in vervet monkeys. Psychopharmacology 136:1–7
Pardridge WM (1983) Brain metabolism: a perspective from the blood brain barrier. Physiol Rev 63:1481–1535
Ramage AG, Fozard JR (1987) Evidence that the putative 5-HT1A receptor agonists, 8-OH-DPAT and ipsapirone, have a central hypotensive action that differs from that of clonidine in anaesthetised cats. Eur J Pharmacol 138:179–191
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Schaechter JD, Wurtman RJ (1990) Serotonin release varies with brain tryptophan levels. Brain Res 532:203-210
Schmitt JAJ, Jorissen BL, Sobezak S, van Boxtel MPJ, Hogervorst E, Deutz NEP, Riedel WJ (2000) Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacology 14:21–29
Soares DD, Coimbra CC, Marubayashi U (2007) Tryptophan-induced central fatigue in exercising rats is related to serotonin content in preoptic area. Neurosci Lett 415:274–278
Stensrud T, Ingjer F, Holm H, Stromme SB (1992) l-tryptophan supplementation does not improve running performance. Int J Sports Med 13:481–485
Stepto NK, Shipperd BB, Hyman G, McInerney B, Pyne DB (2011) Effects of high-dose large neutral amino acid supplementation on exercise, motor skill, and mental performance in Australian rules football players. Appl Physiol Nutr Metab 36:671–681
Strachan AT, Leiper JB, Maughan RJ (2005) Serotonin2C receptor blockade and thermoregulation during exercise in the heat. Med Sci Sport Exerc 37:389–394
Struder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K (1998) Influence of paroxetine, branched-chain amino acids, and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res 30:188–194
Van der Veen FM, Mies GW, Van der Molen MW, Evers EA (2008) Acute tryptophan depletion in healthy males attenuates phasic cardiac slowing but does not affect electro-cortical response to negative feedback. Psychopharmacology 199:255–263
Van Hall G, Raaymakers JSH, Saris WHM, Wagenmakers AJM (1995) Ingestion of branched-chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J Physiol 486:789–794
Watson P (2008) Nutrition, the brain and prolonged exercise. Eur J Sport Sci 8:87–96
Watson P, Shirreffs SM, Maughan RJ (2004) The effect of acute branched chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 93:306–314
Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R (2005) Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 565:873–883
Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila M (1999) Cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem 72:1641–1647
Williams WA, Carson RE, Momenan R, Lang L, Kerich M, Heinz A, Bjork J, Geyer C, Rawlings R, Phillips M, Johnson B, Eckelman WC, Hommer D, Herscovitch P (2002) Effects of acute plasma tryptophan depletion on serotonin receptor occupancy using PET in healthy human subjects. Neuroscience: 826–824
Wilson WM, Maughan RJ (1992) Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol 77:921–924
Yildiz O, Smith JR, Purdy RE (1998) Serotonin and vasoconstrictor synergism. Life Sci 62:1723–1732
Conflict of interest
No finding was received to support this work and the authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hobson, R.M., Watson, P. & Maughan, R.J. Acute tryptophan depletion does not improve endurance cycling capacity in a warm environment. Amino Acids 44, 983–991 (2013). https://doi.org/10.1007/s00726-012-1429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1429-1