Abstract
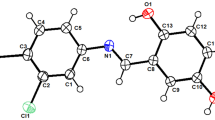
Two novel cyclic hexapeptides, designated NW-G10 (1) and NW-G11 (2), were isolated from the fermentation broth of Streptomyces alboflavus 313. Their relative structures were elucidated on the basis of extensive spectroscopic analysis, and the absolute configurations of several constituent amino acids were determined by Marfey’s method. NW-G10 (1) and NW-G11 (2) exhibited significant activity against Gram-positive bacteria, such as Bacillus cereus, Bacillus subtilis and Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus (MRSA), but they are not active against gram negatives.



Similar content being viewed by others
References
Fujii K, Ikai Y, Mayumi T et al (1997) A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: elucidation of limitations of Marfey’s method and of its separation mechanism. Anal Chem 69:3346–3352
Guo ZY, Shen L, Ji ZQ et al (2009) NW-G01, a novel cyclic hexadepsipeptide antibiotic, produced by Streptomyces alboflavus 313: I. Taxonomy, fermentation, isolation, physicochemical properties and antibacterial activities NW-G01 produced by Streptomyces alboflavus 313. J Antibiot 62:201–205
Guo ZY, Ji ZQ, Zhang JW et al (2010) NW-G01, a novel cyclic hexapeptide antibiotic, produced by Streptomyces alboflavus 313: II. Structural elucidation. J Antibiot 63:231–235
Guo ZY, Shen L, Zhang JW et al (2011) NW-G03, a related cyclic hexapeptide compound of NW-G01, produced by Streptomyces alboflavus 313. J Antibiot 64:789–794
Hassall CH, Magnus KE (1959) Monamycin: a new antibiotic. Nature 184:1223–1224
Huang X, Roemer E, Sattler I et al (2006) Lydiamycins A–D: cyclodepsipetides with antimycobacterial properties. Angew Chem Int Ed 45:3067–3072
Ji ZQ, Wang MA, Zhang JW et al (2007) Two new members of streptothricin class antibiotics from Streptomyces qinlingensis sp. nov. J Antibiot 60:739–744
Kamenecka TM, Danishefsky SJ (1998a) Studies in the total synthesis of himastatin: a revision of the stereochemical assignment. Angew Chem Int Ed 37:2993–2995
Kamenecka TM, Danishefsky SJ (1998b) Total synthesis of himastatin: confirmation of the revised stereostructure. Angew Chem Int Ed 37:2995–2998
Konishi M, Ohkuma H, Sakai F et al (1981) Structures of BBM-928 A, B, and C novel antitumor antibiotics from Actinomadura luzonensis. J Am Chem Soc 103:1241–1243
Lam KS, Hesler GA, Mattei JM et al (1990) Himastatin, a new antitumor antibiotic from Streptomyces hygroscopicus I. Taxonomy of producing organism, fermentation and biological activity. J Antibiot 43:956–960
Leet JE, Schroeder DR, Krishnan BS et al (1990) Himastatin, a new antitumor antibiotic from Streptomyces hygroscopicus II. Isolation and characterization. J Antibiot 43:961–966
Leet JE, Schroeder DR, Golik J et al (1996) Himastatin, a new antitumor antibiotic from Streptomyces hygroscopicus III. Structural elucidation. J Antibiot 49:299–311
Maehr H, Liu CM, Palleroni NJ et al (1986) Microbial products VIII. Azinothricin, a novel hexadepsipeptide antibiotic. J Antibiot 39:17–25
Maskey RP, Fotso S, Sevvana M et al (2006) Kettapeptin: isolation, structure elucidation and activity of a new hexadepsipeptide antibiotic from a terrestrial Streptomyces sp. J Antibiot 59:309–314
Miller ED, Kauffman CA, Jensen PR et al (2007) Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus Streptomyces. J Org Chem 72:323–330
Oelke AJ, France DJ, Hofmann T et al (2010) Total synthesis of chloptosin. Angew Chem Int Ed 49:6139–6142
Oelke AJ, Antonietti F, Bertone L et al (2011) Total synthesis of chloptosin: A dimeric cyclohexapeptide. Chem Eur J 17:4183–4194
Sakai Y, Yoshida T, Tsujita T et al (1997) GE3, a novel hexadepsipeptide antitumor antibiotic, produced by Streptomyces sp. I. Taxonomy, production, isolation, physico-chemical properties, and biological activities. J Antibiot 50:659–664
Setsuya S, Takaaki M, Keisuke T et al (2011) Total synthesis of NW-G01, a cyclic hexapeptide antibiotic, and 34-epi-NW-G01. Org Lett 13:4700–4703
Shimada N, Morimoto K, Naganawa H et al (1981) Antrimycin, a new peptide antibiotic. J Antibiot 34:1613–1614
Smitka TA, Deeter JB, Hunt AH et al (1988) A83586c, a new depsipeptide antibiotic. J Antibiot 41:726–733
Umezawa K, Ikeda Y, Uchihata Y et al (2000) Chloptosin, an apoptosis-inducing dimeric cyclohexapeptide produced by Streptomyces. J Org Chem 65:459–463
Yu SM, Hong WX, Wu Y et al (2010) Total synthesis of chloptosin, a potent apoptosis-inducing cyclopeptide. Org Lett 12:1124–1127
Acknowledgments
This study was supported part by the grant of The National Key Basic Research Program (973 Program, 2010CB126100) from Science and Technology Ministry of China, the National Natural Science Foundation of China (No. 30971935), Postdoctoral Science Foundation of China (20100471644 and 201104683), and Program for New Century Excellent Talents in University from Education Ministry of China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, S., Fan, L., Wu, W. et al. Two piperazic acid-containing cyclic hexapeptides from Streptomyces alboflavus 313. Amino Acids 43, 2191–2198 (2012). https://doi.org/10.1007/s00726-012-1303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1303-1