Abstract
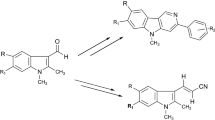
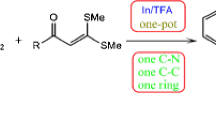
Strategically a new approach for the synthesis tetrahydro-β-carboline unit with the aid of diethyl acetamidomalonate as a glycine equivalent has been described.





Similar content being viewed by others
References
Bailey PD, Hollinshead SP (1988) Application of a modified Pictet–Spengler reaction to the synthesis of optically active tetrahydro-β-carbolines, key intermediates in the preparation of many indole alkaloids. J Chem Soc Perkin Trans 1:739–745
Chopra RN, Gupta JC, Mukherjee B (1933) The pharmacological action of an alkaloid obtained from Rauvolfia serpentina. Indian J Med Res 21:261–271
Cook JM, Cox ED (1995) The Pictet–Spengler condensation: a new direction for an old reaction. Chem Rev 95:1797–1842 (and references cited therein)
Cook JM, Yin W, Kabir MS, Wang Z, Rallapalli SK, Ma J (2010) Enantiospecific total synthesis of the important biogenetic intermediates along the ajmaline pathway, (+)-polyneuridine and (+)-polyneuridine aldehyde, as well as 16-epivellosimine and macusine A. J Org Chem 75:3339–3349
Fujii N, Ohno H, Oishi S, Ohta Y (2009) Facile synthesis of 1,2,3,4-tetrahydro-β-carbolines by one-pot domino three-component indole formation and nucleophilic cyclization. Org Lett 11:1979–1982
Ho TL, Lin QX (2008) Stereoselective synthesis of (±)-tacamonine. Tetrahedron 64:10401–10405
Jenkins PR, Wilson J, Emmerson D, Garcia MD, Smith MR, Gray SJ, Britton RG, Mahale S, Chaudhuri B (2008) Design, synthesis and biological evaluation of new tryptamine and tetrahydro-β-carboline-based selective inhibitors of CDK4. Bioorg Med Chem 16:7728–7739
Kotha S (2003) The building block approach to unusual α-amino acid derivatives and peptides. Acc Chem Res 36:342–351
Kotha S, Halder S (2010) Ethyl isocyanoacetate as useful glycine equivalent. Synlett, 337–354
Kotha S, Singh K (2004) N-Alkylation of diethyl acetamidomalonate: synthesis of constrained amino acid derivatives by ring-closing metathesis reaction. Tetrahedron Lett 45:9607–9610
Kotha S, Misra S, Krishna NG, Devunuri N, Hopf H, Keecherikunne A (2010) Diversity oriented approach to 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) derivatives using diethyl acetamidomalonate as a glycine equivalent. Further explansion by Suzuki–Miyaura cross-coupling reaction. Heterocycles 49:847–852
Liu Z, Xu F (1989) Total synthesis of N a-methyl-Δ18-isokoumidine, a possible precursor of the koumine type indole alkaloids. Tetrahedron Lett 30:3457–3460
Ma J, Yin W, Zhou H, Cook JM (2007) Total synthesis of the opioid agonistic indole alkaloid mitragynine and the first total syntheses of 9-methoxygeissoschizol and 9-methoxy-N b-methylgeissoschizol. Org Lett 9:3491–3494
Magnus P, Gazzard L, Hobson L, Payne AH, Lynch V (1999) Studies on the synthesis of the indole alkaloids pauciflorine A and B. Tetrahedron Lett 40:5135–5138
Mergott DJ, Zuend SJ, Jacobsen EN (2008) Catalytic asymmetric total synthesis of (+)-yohimbine. Org Lett 10:745–748
Moody CJ, Roffey JRA (2000) Synthesis of N-protected nortopsentins B and D. ARKIVOC 3:393–401
Moody CJ, Jackson PM, Harrison CA, Williams MJ (1995) Cyclopenta [b] indoles. Part 2. Model studies towards the tremorgenic mycotoxins. J Chem Soc Perkin Trans 1:1131–1136
Petter A, Engelmann K (1974) Antiarrhythmic action of ajmaline on the heart. Arzneim Forsch 24:876–880
Pfeffer FM, Stewart SG, Priebbenow DL, Henderson LC (2010) Domino Heck–Aza-Michael reactions: efficient access to 1-substituted tetrahydro-β-carbolines. J Org Chem 75:1787–1790
Shen Ya-C, Chen CY, Hsieh PW, Duh CY, Lin YM, Ko CL (2005) The preparation and evaluation of 1-substituted 1,2,3,4-tetrahydro- and 3,4-dihydro-β-carboline derivatives as potential antitumor agents. Chem Pharm Bull 53:32–36
Shipman M, Hayes JF, Tarver GJ, Shiers JJ, Mumford PM (2008) Synthesis of 1,1-disubstituted tetrahydro-b-carbolines from 2-methyleneaziridines. Tetrahedron Lett 24:3489–3491
Soderberg BCG, Dantale SW (2003) A novel palladium-catalyzed synthesis of β-carbolines: application in total synthesis of naturally occurring alkaloids. Tetrahedron 59:5507–5514
Srinivasan PC, Saroja B (1984) A simple route to indole-2,3-quinodimethane-A facile synthesis of carbazoles. Tetrahedron Lett 25:5429–5430
Szawkało J, Czarnocki SJ, Zawadzka A, Wojtasiewicz K, Leniewski A, Maurin JK, Czarnocki Z, Drabowicz J (2007) Enantioselective synthesis of some tetrahydroisoquinoline and tetrahydro-β-carboline alkaloids. Tetrahedron Asymmetry 18:406–413
Verma PP, Sherigara BS, Mahadevan KM, Hulikal VK (2009) Efficient and straightforward synthesis of tetrahydrocarbazoles and 2,3-dimethylindoles catalyzed by CAN. Synth Commun 39:158–165
Acknowledgments
We are grateful to DST and CSIR for the financial support and SAIF Mumbai for providing the spectral data. S. M. thank CSIR (New Delhi) for the award of research fellowship. S. K. thank DST for the award of J. C. Bose fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotha, S., Misra, S. & Mobin, S.M. A new approach to 3-substituted tetrahydro-β-carboline derivative via diethyl acetamidomalonate. Amino Acids 41, 933–936 (2011). https://doi.org/10.1007/s00726-010-0792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0792-z