Abstract
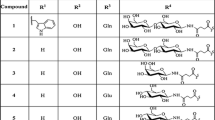
Bioconjugates with receptor-mediated tumor-targeting functions and carrying cytotoxic agents should enable the specific delivery of chemotherapeutics to malignant tissues, thus increasing their local efficacy while limiting the peripheral toxicity. In the present study, gonadotropin-releasing hormone III (GnRH-III; Glp-His-Trp-Ser-His-Asp-Trp-Lys-Pro-Gly-NH2) was employed as a targeting moiety to which daunorubicin was attached via oxime bond, either directly or by insertion of a GFLG or YRRL tetrapeptide spacer. The in vitro antitumor activity of the bioconjugates was determined on MCF-7 human breast and HT-29 human colon cancer cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Their degradation/stability (1) in human serum, (2) in the presence of cathepsin B and (3) in rat liver lysosomal homogenate was analyzed by liquid chromatography in combination with mass spectrometry. The results show that (1) all synthesized bioconjugates have in vitro antitumor effect, (2) they are stable in human serum at least for 24 h, except for the compound containing an YRRL spacer and (3) they are hydrolyzed by cathepsin B and in the lysosomal homogenate. To investigate the relationship between the in vitro antitumor activity and the structure of the bioconjugates, the smallest metabolites produced in the lysosomal homogenate were synthesized and their binding to DNA was assessed by fluorescence spectroscopy. Our data indicate that the incorporation of a peptide spacer in the structure of oxime bond-linked daunorubicin–GnRH-III bioconjugates is not required for their antitumor activity. Moreover, the antitumor activity is influenced by the structure of the metabolites (daunorubicin–amino acid derivatives) and their DNA-binding properties.






Similar content being viewed by others
References
Bai KB, Láng O, Orbán E, Szabó R, Kőhidai L, Hudecz F, Mező G (2008) Design, synthesis, and in vitro activity of novel drug delivery systems containing tuftsin derivatives and methotrexate. Bioconjug Chem 19:2260–2269
Bajusz S, Janáky T, Csernus VJ, Bokser L, Fekete M, Srkalovic G, Redding TW, Schally AV (1989) Highly potent metallopeptide analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA 86:6313–6317
Barcelo F, Martorell J, Gavilanes F, Gonzalez-Ros JM (1988) Equilibrium binding of daunomycin and adriamycin to calf thymus DNA. Temperature and ionic strength dependence of thermodynamic parameters. Biochem Pharmacol 37:2133–2138
Bax A, Davis DG (1985) MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson 65:355–360
Braslawsky GR, Kadow K, Knipe J, McGoff K, Edson M, Kaneko T, Greenfields RS (1991) Adriamycin(hydrazone)-antibody conjugates require internalization and intracellular acid hydrolysis for antitumor activity. Cancer Immunol Immunother 33:367–374
Buré C, Levièvre D, Delmas A (2000) Identification of by-products from an orthogonal peptide ligation by oxime bonds using mass spectrometry and tandem mass spectrometry. Rapid Commun Mass Spectrom 14:2158–2164
Chaires JB (1990) Biophysical chemistry of the daunomycin–DNA interaction. Biophys Chem 35:191–202
Chaires JB, Dattagupta N, Crothers DM (1982) Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry 21:3933–3940
Collins SJ, Gallo RC, Gallagher RE (1977) Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 270:347–349
David A, Kopeckova P, Minko T, Rubinstein A, Kopecek J (2004) Design of a multivalent galactoside ligand for selective targeting of HPMA copolymer-doxorubicin conjugates to human colon cancer cells. Eur J Cancer 40:148–157
Dubowchik GM, Firestone RA (1998) Cathepsin B-sensitive dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (Taxol), mitomycin C and doxorubicin. Bioorg Med Chem Lett 8:3341–3346
Dubowchik GM, Walker MA (1999) Receptor-mediated and enzyme-dependent targeting of cytotoxic anticancer drugs. Pharmacol Ther 83:67–123
Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA (2002) Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem 13:855–869
Etrych T, Jelínková M, Ríhová B, Ulbrich K (2001) New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release 73:89–102
Gilladoga AC, Manuel C, Tan CT, Wollner N, Sternberg SS, Murphy ML (1976) The cardiotoxicity of adriamycin and daunomycin in children. Cancer 37:1070–1078
Graves DE, Krugh TR (1983) Adriamycin and daunorubicin bind in a cooperative manner to deoxyribonucleic acid. Biochemistry 22:3941–3947
Herédi-Szabó K, Lubke J, Tóth G, Murphy RF, Lovas S (2005) Importance of the central region of lamprey gonadotropin-releasing hormone III in the inhibition of breast cancer cell growth. Peptides 26:419–422
Herédi-Szabó K, Murphy RF, Lovas S (2006) Different signal response to lamprey GnRH-III in human cancer cells. Int J Pept Res Ther 12:359–364
Hwang T-L, Shaka AJ (1995) Water suppression that works. Excitation sculpting using arbitrary waveforms and pulsed field gradients. J Magn Reson Ser A 112:275–279
Jaracz S, Chen J, Kuznetsova LV, Ojima I (2005) Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem 13:5043–5450
Keller R (2004) The computer aided resonance assignment tutorial, 1st edn. Cantina Verlag
Kim BS, Moon SS, Hwang BK (2000) Structure elucidation and antifungal activity of an anthracycline antibiotic, daunomycin, isolated from Sctinomadura roseola. J Agric Food Chem 48:1875–1881
Kovács M, Vincze B, Horváth JE, Seprődi J (2007) Structure–activity study on the LH- and FSH-releasing and anticancer effects of gonadotropin-releasing hormone (GnRH)-III analogs. Peptides 28:821–829
Kovács M, Szepesházi K, Schally AV (2009) Endocrine and antineoplastic effects ofantagonistic and cytotoxic analogs of luteinising hormone-releasing hormone. In: Kovács M, Merchenhalter I (eds) Neuropeptides and peptide analogs. Research Signpost, Kerela, India, pp 33–57
Krohn K (2008) Interaction of natural and synthetic anthracyclines with DNA. Curr Bioact Compounds 4:175–188
Luo Y, Bernshaw NJ, Lu ZR, Kopecek J, Prestwich GD (2002) Targeted delivery of doxorubicin by HPMA copolymer–hyaluronan bioconjugates. Pharm Res 19:396–402
Malugin A, Kopecková P, Kopecek J (2007) Liberation of doxorubicin from HPMA copolymer conjugate is essential for the induction of cell cycle arrest and nuclear fragmentation in ovarian carcinoma cells. J Control Release 124:6–10
McGhee JD, Von Hippel PH (1974) Theoretical aspects of DNA protein interactions: cooperative and non cooperative binding of large ligands to a one dimensional homogeneous lattice. J Mol Biol 86:469–489
Ménard R, Carmona E, Plouffe C, Brömme D, Konishi Y, Lefebvre J, Storer AC (1993) The specificity of the S1’ subsite of cysteine proteases. FEBS Lett 328:107–110
Mező G, Manea M (2010) Receptor-mediated tumor targeting based on peptide hormones. Expert Opin Drug Deliv 7:79–96
Mező I, Lovas S, Pályi I, Vincze B, Kálnay A, Turi G, Vadász Zs, Seprődi J, Idei M, Tóth G, Gulyás É, Ötvös F, Mák M, Horváth JE, Teplán I, Murphy RF (1997) Synthesis of gonadotropin-releasing hormone III analogs. Structure–antitumor activity relationships. J Med Chem 40:3353–3358
Mező G, Czajlik A, Manea M, Jakab A, Farkas V, Majer Z, Vass E, Bodor A, Kapuvári B, Boldizsár M, Vincze B, Csuka O, Perczel A, Przybylski M, Hudecz F (2007) Structure, enzymatic stability and antitumor activity of sea lamprey GnRH-III and its dimer derivatives. Peptides 28:806–820
Mező G, Manea M, Szabó I, Vincze B, Kovács M (2008) New derivatives of GnRH as potential anticancer therapeutic agents. Curr Med Chem 15:2366–2379
Mező G, Szabó I, Kertész I, Hegedüs R, Orbán E, Leurs U, Bősze S, Halmos G, Manea M (2010) Efficient synthesis of an (aminooxy)acetyled somatostatin derivative using (aminooxy)acetic acid as a „carbonyl capture” reagent. J Pept Sci. doi:10.1002/psc.1294
Minotti G, Cavaliere AF, Mordente A, Rossi M, Schiavello R, Zamparelli M, Possati G-F (1995) Secondary alcohol metabolites mediate iron delocalization in cytosolic fractions of myocardial biopsies exposed to anticancer anthracyclines. Novel linkage between anthracycline metabolism and iron-induced cardiotoxicity. J Clin Invest 95:1595–1605
Nagy A, Schally AV, Armatis P, Szepesházi K, Halmos G, Kovács M, Zarándi M, Groot K, Miyazaka M, Jungwirth A, Horváth J (1996) Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500–1000 times more potent. Proc Natl Acad Sci USA 93:7269–7273
Nagy A, Plonowski A, Schally AV (2000) Stability of cytotoxic luteinizing hormone-releasing hormone conjugate (AN-152) containing doxorubicin 14-O-hemiglutarate in mouse and human serum in vitro: implications for the design of preclinical studies. Proc Natl Acad Sci USA 97:829–834
Omelyanenko V, Gentry C, Kopecková P, Kopecek J (1998) HPMA copolymer-anticancer drug-OV-TL16 antibody conjugates. II. Processing in epithelial ovarian carcinoma cells in vitro. Int J Cancer 75:600–608
Pályi I, Vincze B, Lovas S, Mező I, Pató J, Kálnai A, Túri G, Gaál D, Mihalik R, Péter I, Teplán I, Murphy RF (1999) Gonadotropin-releasing hormone analogue conjugates with strong selective antitumor activity. Proc Natl Acad Sci USA 96:2361–2366
Parthiban P, Balasubramanian S, Adrioss G, Kabilan S (2008) Synthesis and NMR spectral studies of some 2, 6-diarylpiperidin-4-one O-benzyloximes. Spectrochim Acta A Mol Biomol Spectrosc 70:11–24
Schally AV, Nagy A (2003) New approaches to treatment of various cancers based on cytotoxic analogs of LHRH, somatostatin and bombesin. Life Sci 72:2305–2320
Schally AV, Nagy A (2004) Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab 15:300–310
Shao J, Tam JP (1995) Unprotected peptides as building blocks for the synthesis of peptide dendrimers with oxime, hydrazone, and thiazolidine linkages. J Am Chem Soc 117:3893–3899
Sibrian-Vazquez M, Jensen TJ, Vicente MG (2008) Synthesis, characterization, and metabolic stability of porphyrin–peptide conjugates bearing bifunctional signaling sequences. J Med Chem 51:2915–2923
Singh Y, Palombo M, Sinko PJ (2008) Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem 15:1802–1826
Sower SA, Chiang Y-C, Lovas S, Conlon JM (1993) Primary structure and biological activity of a third gonadotropin-releasing hormone from lamprey brain. Endocrinology 132:1125–1131
Szabó I, Manea M, Orbán E, Csámpai A, Bősze S, Szabó R, Tejeda M, Gaál D, Kapuvári B, Przybylski M, Hudecz F, Mező G (2009) Development of an oxime bond containing daunorubicin–gonadotropin-releasing hormone-III conjugate as a potential anticancer drug. Bioconjug Chem 20:656–665
Taralp A, Kaplan H, Sytwu II, Vlattas I, Bohacek R, Knap AK, Hirama T, Huber CP, Hasnain S (1995) Characterization of the S3 subsite specificity of cathepsin B. J Biol Chem 270:18036–18043
Trail PA, King HD, Dubowchik GW (2003) Monoclonal antibody drug immunoconjugates for targeted treatment of cancer. Cancer Immunol Immunother 52:328–337
Valentini L, Nicolella V, Vannini E (1985) Association of anthracycline derivatives with DNA: a fluorescence study. Farmaco Sci 40:377–390
Yadav AK, Mishra P, Agrawal GP (2008) An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target 16:91–107
Yamazaki N, Kojima S, Bovin NV, Andre S, Gabius S, Gabius HJ (2000) Endogenous lectins as targets for drug delivery. Adv Drug Deliv Rev 43:225–244
Acknowledgments
This work was supported by Grants from the University of Konstanz (Zukunftskolleg, Project 879/08 and AFF, Project 836/09), the Hungarian National Science Fund (OTKA NK 77485) and GVOP-3.2.1.-2004-04-0005/3. The authors thank the ChemAxon Kft. (Budapest, Hungary) for the MarvinSketch Version 5.2 software.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Orbán, E., Mező, G., Schlage, P. et al. In vitro degradation and antitumor activity of oxime bond-linked daunorubicin–GnRH-III bioconjugates and DNA-binding properties of daunorubicin–amino acid metabolites. Amino Acids 41, 469–483 (2011). https://doi.org/10.1007/s00726-010-0766-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0766-1