Abstract
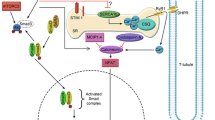
Depletion of skeletal muscle protein mainly results from enhanced protein breakdown, caused by activation of proteolytic systems such as the Ca2+-dependent and the ATP-ubiquitin-dependent ones. In the last few years, enhanced expression and bioactivity of myostatin have been reported in several pathologies characterized by marked skeletal muscle depletion. More recently, high myostatin levels have been associated with glucocorticoid-induced hypercatabolism. The search for therapeutical strategies aimed at preventing/correcting protein hypercatabolism has been directed to inhibit humoral mediators known for their pro-catabolic action, such as TNFα. The present study has been aimed to investigate the involvement of TNFα in the regulation of both myostatin expression and intracellular protein catabolism, and the possibility to interfere with such modulations by means of amino acid supplementation. For this purpose, C2C12 myotubes exposed to TNFα in the presence or in the absence of amino acid (glutamine or leucine) supplementation have been used. Myotube treatment with TNFα leads to both hyperexpression of the muscle-specific ubiquitin ligase atrogin-1, and enhanced activity of the Ca2+-dependent proteolytic system. These changes are associated with increased myostatin expression. Glutamine supplementation effectively prevents TNFα-induced muscle protein loss and restores normal myostatin levels. The results shown in the present study indicate a direct involvement of TNFα in the onset of myotube protein loss and in the perturbation of myostatin-dependent signaling. In addition, the protective effect exerted by glutamine suggests that amino acid supplementation could represent a possible strategy to improve muscle mass.






Similar content being viewed by others
References
Ahmad S, Karlstad MD, Choudhry MA et al (1994) Sepsis-induced myofibrillar protein catabolism in rat skeletal muscle. Life Sci 55:1383–1391
Argiles JM, Lopez-Soriano FJ (1999) The role of cytokines in cancer cachexia. Med Res Rev 19:223–248
Argilés JM, Busquets S, Toledo M et al (2009) The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3:263–268
Baracos VE (2001) Management of muscle wasting in cancer-associated cachexia. Understanding gained from experimental studies. Cancer Suppl 92:1669–1677
Beyette J, Mason GGF, Murray RZ et al (1998) Proteasome activities decrease during dexamethasone-induced apoptosis of thymocytes. Biochem J 332:315–320
Bodine SC, Stitt TN, Gonzalez M et al (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Bogdanovich S, Krag TO, Barton ER et al (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418–421
Bonetto A, Penna F, Minero VG et al (2009) Deacetylase inhibitors modulate the myostatin/follistatin axis without improving cachexia in tumor-bearing mice. Curr Cancer Drug Targets 9:608–616
Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15:1753–1765
Burattini S, Ferri P, Battistelli M et al (2004) C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem 48:223–233
Cai D, Frantz JD, Tawa NE Jr et al (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298
Capel F, Prodhomme M, Béchet D et al (2009) Lysosomal and proteasome-dependent proteolysis are differentially regulated by insulin and/or amino acids following feeding in young, mature and old rats. J Nutr Biochem 20(8):570–576
Clop A, Marcq F, Takeda H et al (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheeps. Nat Genet 38:813–818
Costelli P, Baccino FM (2003) Mechanisms of skeletal muscle depletion in wasting syndromes: role of ATP-ubiquitin-dependent proteolysis. Curr Opin Clin Nutr Metab Care 6:407–412
Costelli P, Carbó N, Tessitore L et al (1993) Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 92:2783–2789
Costelli P, Bossola M, Muscaritoli M et al (2002) Anti-cytokine treatment prevents the increase in the activity of ATP-ubiquitin- and Ca2+-dependent proteolytic systems in the muscle of tumour-bearing rats. Cytokine 19:1–5
Costelli P, Muscaritoli M, Bossola M et al (2006) IGF-1 is reduced in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 29:R674–R683
Costelli P, Muscaritoli M, Bonetto A et al (2008) Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest 38:531–538
Dillon EL, Sheffield-Moore M, Paddon-Jones D et al (2009) Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab 94:1630–1637
Du M, Shen QW, Zhu MJ et al (2007) Leucine stimulate mammalian target of rapamycin signalling in C2C12 myoblasts in part trough inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci 85:919–927
Fernandez-Celemin L, Pasko N, Blomart V et al (2002) Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 283:E1279–E1290
Frost RA, Lang CH (2007) Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103:378–387
Gilson H, Schakman O, Combaret L et al (2007) Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 148:452–460
Goll DE, Kleese WC, Szpacenko A (1989) Skeletal muscle proteases and protein turnover. In: Campion DR, Hausman GJ, Martin RJ et al (eds) Animal growth regulation. Plenum, New York
Gomes MD, Lecker SH, Jagoe RT et al (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98:14440–14445
Gonzalez-Cadavid NF, Taylor WE, Yarasheski K et al (1998) Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA 95:14938–14943
Guttridge DC (2004) Signaling pathways weigh on decision to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care 7:443–450
Herningtyas EH, Okimura Y, Handayaningsih AE et al (2008) Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim Biophys Acta 1780:1115–1120
Hickson RC, Czerwinski SM, Wegrzyn LE (1995) Glutamine prevents downregulation of myosin heavy chain synthesis and muscle atrophy from GCs. Am J Physiol 268:E730–E734
Holzbaur EL, Howland DS, Weber N et al (2006) Myostatin inhibition slows muscle atrophy in rodent models of amyotrophic lateral sclerosis. Neurobiol Dis 23:697–707
Hoshino E, Pichard C, Greenwood CE et al (1991) Body composition and metabolic rate in rat during a continuous infusion of cachectin. Am J Physiol 260:E27–E36
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834–C843
Lang CH, Frost RA, Nairn AC et al (2002) TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab 282:E336–E347
Lecker SH, Solomon V, Price SR et al (1999) Ubiquitin conjugation by the N-end rule pathway and mRNAs for its components increase in muscles of diabetic rats. J Clin Invest 104:1411–1420
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311
Lenk K, Schur R, Linke A et al (2009) Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail 11:342–348
Li YP, Reid MB (2000) NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 279:R1165–R1170
Li YP, Schwartz RJ, Waddell ID et al (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12:871–880
Li YP, Lecker SH, Chen Y et al (2003) TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17:1048–1057
Li YP, Chen Y, John J et al (2005) TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19:362–370
Li Y, Li Y, Feng Q et al (2009) Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocites. Cardiovasc Res 83(1):100–110
Liu C-M, Yang Z, Liu C-W et al (2008) Myostatin antisense RNA-mediated muscle growth in normal and cancer cachexia mice. Gene Ther 15:155–160
Louard RJ, Barrett EJ, Gelfand RA (1995) Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 44:424–429
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma K, Mallidis C, Bhasin S et al (2003) Glucocorticoid-induced skeletal muscle atrophy is associated with up-regulation of myostatin gene expression. Am J Physiol Endocrinol Metab 285:E363–E371
Martinet W, De Meyer GRY, Herman AG et al (2005) Amino acid deprivation induces both apoptosis and autophagy in murine C2C12 muscle cells. Biotechnol Lett 27:1157–1163
McFarlane C, Plummer E, Thomas M et al (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Meador BM, Huey KA (2009) Glutamine preserves skeletal muscle force during an inflammatory insult. Muscle Nerve 40:1000–1007
Moylan JS, Smith JD, Chambers MA et al (2008) TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295:C986–C993
Nair KS, Schwartz RG, Welle S (1992) Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol Endocrinol Metab 263:E928–E934
Penna F, Bonetto A, Muscaritoli M et al. (2010) Muscle atrophy in experimental cancer cachexia: Is the IGF-1 signaling pathway involved? Int J Cancer (in press)
Rebbapragada A, Benchabane H, Wrana JL et al (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7742
Robinson LE, Bussiere FI, Le Boucher J et al (1999) Amino acid nutrition and immune function in tumor-bearing rats: a comparison of glutamine-, arginine- and ornithine 2-oxoglutarate-supplemented diets. Clin Sci 97:657–669
Ruiz-Vela A, Gonzales de Buitrago G, Martinez AC (1999) Implication of calpain in caspase activation during B cell clonal deletion. EMBO J 18:4988–4998
Salehian B, Mahabadi V, Bilas J et al (2006) The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 55:1239–1247
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology 23:160–170
Solerte SB, Gazzaruso C, Bonacasa R et al (2008) Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol 101:69E–77E
Sugawara T, Ito Y, Nishizawa N et al (2009) Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 37:609–616
Talvas J, Obled A, Fafournoux P et al (2006) Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 136:1466–1471
Ventadour S, Attaix D (2006) Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18:631–665
Wiemer AJ, Lokuta MA, Surfus JC et al (2010) Calpain inhibition impairs TNF-alpha-mediated neutrophil adhesion, arrest and oxidative burst. Mol Immunol 47:894–902
Zhu X, Topouzis S, Liang LF et al (2004) Myostatin signalling through Sma2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 26:262–272
Zimmers TA, Davies MV, Koniaris LG et al (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488
Acknowledgments
Work supported by ‘Ministero per l’Università e la Ricerca’ (MIUR, Roma; PRIN projects), University of Torino (ex-60% funds), Regione Piemonte, Compagnia di San Paolo (Torino) and Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonetto, A., Penna, F., Minero, V.G. et al. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-α. Amino Acids 40, 585–594 (2011). https://doi.org/10.1007/s00726-010-0683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0683-3